Beyond Borders: Global biotechnology report 2010
Beyond Borders: Global biotechnology report 2010
Beyond Borders: Global biotechnology report 2010
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
the beginning of the year to nearly US$3.5<br />
billion (US$26.40/share) by year-end.<br />
Dendreon expects a complete response<br />
letter from the FDA in <strong>2010</strong>.<br />
Human Genome Sciences (HGS) received<br />
a similar boost from positive pipeline<br />
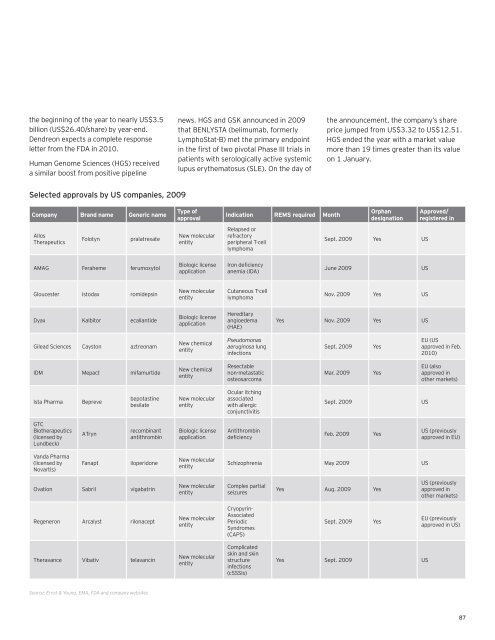
Selected approvals by US companies, 2009<br />
Company Brand name Generic name<br />
Allos<br />
Therapeutics<br />
Folotyn pralatrexate<br />
AMAG Feraheme ferumoxytol<br />
Gloucester Istodax romidepsin<br />
Dyax Kalbitor ecallantide<br />
Gilead Sciences Cayston aztreonam<br />
IDM Mepact mifamurtide<br />
Ista Pharma Bepreve<br />
GTC<br />
Biotherapeutics<br />
(licensed by<br />
Lundbeck)<br />
Vanda Pharma<br />
(licensed by<br />
Novartis)<br />
ATryn<br />
bepotastine<br />
besilate<br />
recombinant<br />
antithrombin<br />
Fanapt iloperidone<br />
Ovation Sabril vigabatrin<br />
Regeneron Arcalyst rilonacept<br />
Theravance Vibativ telavancin<br />
Source: Ernst & Young, EMA, FDA and company websites<br />
news. HGS and GSK announced in 2009<br />
that BENLYSTA (belimumab, formerly<br />
LymphoStat-B) met the primary endpoint<br />
in the first of two pivotal Phase III trials in<br />
patients with serologically active systemic<br />
lupus erythematosus (SLE). On the day of<br />
Type of<br />
approval<br />
New molecular<br />
entity<br />
Biologic license<br />
application<br />
New molecular<br />
entity<br />
Biologic license<br />
application<br />
New chemical<br />
entity<br />
New chemical<br />
entity<br />
New molecular<br />
entity<br />
Biologic license<br />
application<br />
New molecular<br />
entity<br />
New molecular<br />
entity<br />
New molecular<br />
entity<br />
New molecular<br />
entity<br />
Indication REMS required Month<br />
Relapsed or<br />
refractory<br />
peripheral T-cell<br />
lymphoma<br />
Iron deficiency<br />
anemia (IDA)<br />
Cutaneous T-cell<br />
lymphoma<br />
Hereditary<br />
angioedema<br />
(HAE)<br />
Pseudomonas<br />
aeruginosa lung<br />
infections<br />
Resectable<br />
non-metastatic<br />
osteosarcoma<br />
Ocular itching<br />
associated<br />
with allergic<br />
conjunctivitis<br />
Antithrombin<br />
deficiency<br />
Orphan<br />
designation<br />
Sept. 2009 Yes US<br />
June 2009 US<br />
Nov. 2009 Yes US<br />
Yes Nov. 2009 Yes US<br />
Sept. 2009 Yes<br />
Mar. 2009 Yes<br />
Sept. 2009 US<br />
Feb. 2009 Yes<br />
Schizophrenia May 2009 US<br />
Complex partial<br />
seizures<br />
Cryopyrin-<br />
Associated<br />
Periodic<br />
Syndromes<br />
(CAPS)<br />
Complicated<br />
skin and skin<br />
structure<br />
infections<br />
(cSSSIs)<br />
the announcement, the company’s share<br />
price jumped from US$3.32 to US$12.51.<br />
HGS ended the year with a market value<br />
more than 19 times greater than its value<br />
on 1 January.<br />
Yes Aug. 2009 Yes<br />
Sept. 2009 Yes<br />
Yes Sept. 2009 US<br />
Approved/<br />
registered in<br />
EU (US<br />
approved in Feb.<br />
<strong>2010</strong>)<br />
EU (also<br />
approved in<br />
other markets)<br />
US (previously<br />
approved in EU)<br />
US (previously<br />
approved in<br />
other markets)<br />
EU (previously<br />
approved in US)<br />
87