Beyond Borders: Global biotechnology report 2010
Beyond Borders: Global biotechnology report 2010
Beyond Borders: Global biotechnology report 2010
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
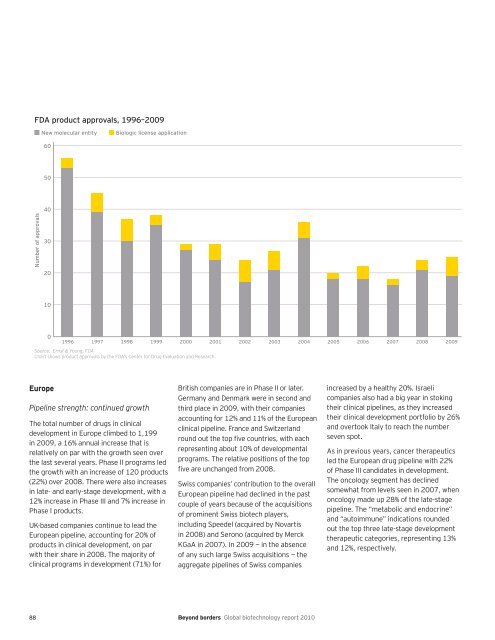
FDA product approvals, 1996–2009<br />
Number of approvals<br />
New molecular entity Biologic license application<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009<br />
Source: Ernst & Young, FDA<br />
Chart shows product approvals by the FDA’s Center for Drug Evaluation and Research.<br />
Europe<br />
Pipeline strength: continued growth<br />
The total number of drugs in clinical<br />
development in Europe climbed to 1,199<br />
in 2009, a 16% annual increase that is<br />
relatively on par with the growth seen over<br />
the last several years. Phase II programs led<br />
the growth with an increase of 120 products<br />
(22%) over 2008. There were also increases<br />
in late- and early-stage development, with a<br />
12% increase in Phase III and 7% increase in<br />
Phase I products.<br />
UK-based companies continue to lead the<br />
European pipeline, accounting for 20% of<br />
products in clinical development, on par<br />
with their share in 2008. The majority of<br />
clinical programs in development (71%) for<br />
British companies are in Phase II or later.<br />
Germany and Denmark were in second and<br />
third place in 2009, with their companies<br />
accounting for 12% and 11% of the European<br />
clinical pipeline. France and Switzerland<br />
round out the top five countries, with each<br />
representing about 10% of developmental<br />
programs. The relative positions of the top<br />
five are unchanged from 2008.<br />
Swiss companies’ contribution to the overall<br />
European pipeline had declined in the past<br />
couple of years because of the acquisitions<br />
of prominent Swiss biotech players,<br />
including Speedel (acquired by Novartis<br />
in 2008) and Serono (acquired by Merck<br />
KGaA in 2007). In 2009 — in the absence<br />
of any such large Swiss acquisitions — the<br />
aggregate pipelines of Swiss companies<br />
88 <strong>Beyond</strong> borders <strong>Global</strong> <strong>biotechnology</strong> <strong>report</strong> <strong>2010</strong><br />
increased by a healthy 20%. Israeli<br />
companies also had a big year in stoking<br />
their clinical pipelines, as they increased<br />
their clinical development portfolio by 26%<br />
and overtook Italy to reach the number<br />
seven spot.<br />
As in previous years, cancer therapeutics<br />
led the European drug pipeline with 22%<br />
of Phase III candidates in development.<br />
The oncology segment has declined<br />
somewhat from levels seen in 2007, when<br />
oncology made up 28% of the late-stage<br />
pipeline. The “metabolic and endocrine”<br />
and “autoimmune” indications rounded<br />
out the top three late-stage development<br />
therapeutic categories, representing 13%<br />
and 12%, respectively.