Зарегистрировано в Минюсте РФ 22 марта 2002 г
Зарегистрировано в Минюсте РФ 22 марта 2002 г
Зарегистрировано в Минюсте РФ 22 марта 2002 г
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
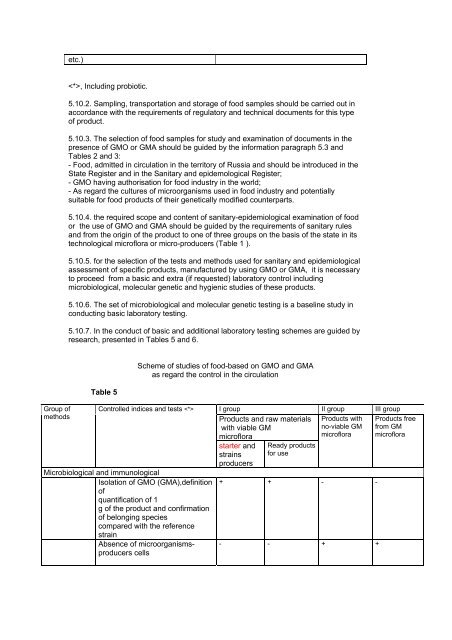
Group of<br />
methods<br />
etc.)<br />
, Including probiotic.<br />
5.10.2. Sampling, transportation and storage of food samples should be carried out in<br />
accordance with the requirements of regulatory and technical documents for this type<br />
of product.<br />
5.10.3. The selection of food samples for study and examination of documents in the<br />
presence of GMO or GMA should be guided by the information paragraph 5.3 and<br />
Tables 2 and 3:<br />
- Food, admitted in circulation in the territory of Russia and should be introduced in the<br />
State Register and in the Sanitary and epidemological Register;<br />
- GMO having authorisation for food industry in the world;<br />
- As regard the cultures of microorganisms used in food industry and potentially<br />
suitable for food products of their genetically modified counterparts.<br />
5.10.4. the required scope and content of sanitary-epidemiological examination of food<br />
or the use of GMO and GMA should be guided by the requirements of sanitary rules<br />
and from the origin of the product to one of three groups on the basis of the state in its<br />
technological microflora or micro-producers (Table 1 ).<br />
5.10.5. for the selection of the tests and methods used for sanitary and epidemiological<br />
assessment of specific products, manufactured by using GMO or GMA, it is necessary<br />
to proceed from a basic and extra (if requested) laboratory control including<br />
microbiological, molecular genetic and hygienic studies of these products.<br />
5.10.6. The set of microbiological and molecular genetic testing is a baseline study in<br />
conducting basic laboratory testing.<br />
5.10.7. In the conduct of basic and additional laboratory testing schemes are guided by<br />
research, presented in Tables 5 and 6.<br />
Table 5<br />
Scheme of studies of food-based on GMO and GMA<br />
as regard the control in the circulation<br />
Controlled indices and tests <br />
Microbiological and immunological<br />
Isolation of GMO (GMA),definition<br />
of<br />
quantification of 1<br />
g of the product and confirmation<br />
of belonging species<br />
compared with the reference<br />
strain<br />
Absence of microorganismsproducers<br />
cells<br />
I group II group III group<br />
Products and raw materials<br />
with viable GM<br />
microflora<br />
starter and<br />
strains<br />
producers<br />
Ready products<br />
for use<br />
Products with<br />
no-viable GM<br />
microflora<br />
+ + - -<br />
- - + +<br />
Products free<br />
from GM<br />
microflora