Abstract Book of EAVLD2012 - eavld congress 2012
Abstract Book of EAVLD2012 - eavld congress 2012
Abstract Book of EAVLD2012 - eavld congress 2012
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
S1 - P - 15<br />
ACCURACY OF PARATUBERCULOSIS ANTIBODY ELISA AT PREDICTING FECAL SHEDDING OF<br />
MYCOBACTERIUM AVIUM SUBSP PARATUBERCULOSIS IN CATTLE<br />
Rakel T. Fernández 1 , Carmen Eiras 2 , Carmen Calvo 2 , Ignacio Arnaiz 2 , Fco. Javier Diéguez 1,3<br />
1<br />
Santiago de Compostela University (Veterinary Faculty), Department <strong>of</strong> Anatomy and Animal Production, Lugo, Spain<br />
2<br />
Animal Health and Production Laboratory, Lugo, Spain<br />
3<br />
Santiago de Compostela University (Veterinary Faculty), Institute <strong>of</strong> Food Analysis and Research, Lugo, Spain<br />
Paratuberculosis, bovine, ELISA, fecal culture<br />
Introduction<br />
Paratuberculosis, also called Johne's disease, is chronic<br />
granulomatous enteritis caused by Mycobacterium avium subsp.<br />
paratuberculosis (MAP). This infectious disease has a worldwide<br />
distribution affecting mainly ruminants, both domestic and wild,<br />
but has also been isolated from other animal species. It causes<br />
great economic losses in cattle farming 1 .<br />
ELISA is an essential tool in paratuberculosis control programs,<br />
although bacterial culture remains the reference confirmatory<br />
test. In this line, the aim <strong>of</strong> this study was to determine the<br />
accuracy <strong>of</strong> the ELISA test as a predictor <strong>of</strong> the MAP fecal<br />
elimination "status" (determined by means <strong>of</strong> fecal culture) <strong>of</strong><br />
individual animals.<br />
Materials & methods<br />
The study was carried out in Galicia (north-west Spain). Galicia is<br />
the major cattle-farming region <strong>of</strong> Spain. It was responsible for<br />
35% <strong>of</strong> the milk and 12% <strong>of</strong> the beef produced in Spain,<br />
constituting approximately 1.7% <strong>of</strong> the milk and 1.3% <strong>of</strong> the beef<br />
produced in the European Union.<br />
Bacteriological culture was performed in fecal samples from 239<br />
animals that were collected during the period 2007-2010. 181<br />
animals were negative to fecal culture and 58 were culture<br />
positive animals. Serological analysis (ELISA) was also<br />
performed in serum samples from the same animals.<br />
Bacterial culture was performed as described by the World<br />
Organization for Animal Health (OIE) (2008) 2 . Briefly, 1 g <strong>of</strong> feces<br />
was added to 20 mL <strong>of</strong> sterile distilled water, and tubes were<br />
shaken for 30 min and then allowed to stand undisturbed for 30<br />
min. Five millilitres <strong>of</strong> the supernatant were added to 0.75%<br />
hexadecylpyridinium chloride (HPC) (Sigma). Tubes were<br />
inverted several times and allowed to stand undisturbed for 18 h<br />
at room temperature for decontamination. Triplicate Herrold’s egg<br />
yolk medium (HEYM) culture slopes containing amphotericin B,<br />
vancomycin and nalidixic acid were inoculated with 0.1 mL <strong>of</strong> the<br />
undisturbed sediment, incubated at 37º C and observed at 2-<br />
week intervals for 16 weeks. Suspect colonies were evaluated for<br />
mycobactin dependence along with morphology and acid-fast<br />
staining. Positive-staining colonies were confirmed by PCR. For<br />
every nine fecal samples a positive control (a positive field<br />
sample) was included.<br />
The ELISA used was “PARATUBERCULOSIS ANTIBODY<br />
SCREENING” (Institute Pourquier, France). False-positive results<br />
were reduced by pre-absorbing the samples with sonicates <strong>of</strong> the<br />
environmental mycobacterium Mycobacterium phlei. Samples<br />
were considered positive at a % sample:positive ratio <strong>of</strong> 55% or<br />
more.<br />
Receiver operating characteristic (ROC) procedure was used to<br />
evaluate the overall diagnostic accuracy to estimate the antibody<br />
titer that was the best cut-<strong>of</strong>f point in terms <strong>of</strong> sensitivity and<br />
specificity as a predictor <strong>of</strong> the bacteriological "status" <strong>of</strong> the<br />
animals (culture positive/negative).<br />
Results<br />
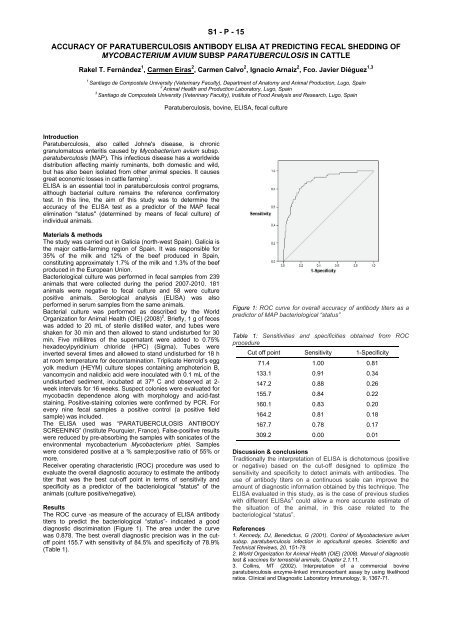
The ROC curve -as measure <strong>of</strong> the accuracy <strong>of</strong> ELISA antibody<br />
titers to predict the bacteriological “status”- indicated a good<br />
diagnostic discrimination (Figure 1). The area under the curve<br />
was 0.878. The best overall diagnostic precision was in the cut<strong>of</strong>f<br />
point 155.7 with sensitivity <strong>of</strong> 84.5% and specificity <strong>of</strong> 78.9%<br />
(Table 1).<br />
Figure 1: ROC curve for overall accuracy <strong>of</strong> antibody titers as a<br />
predictor <strong>of</strong> MAP bacteriological “status”<br />
Table 1: Sensitivities and specificities obtained from ROC<br />
procedure<br />
Cut <strong>of</strong>f point Sensitivity 1-Specificity<br />
71.4 1.00 0.81<br />
133.1 0.91 0.34<br />
147.2 0.88 0.26<br />
155.7 0.84 0.22<br />
160.1 0.83 0.20<br />
164.2 0.81 0.18<br />
167.7 0.78 0.17<br />
309.2 0.00 0.01<br />
Discussion & conclusions<br />
Traditionally the interpretation <strong>of</strong> ELISA is dichotomous (positive<br />
or negative) based on the cut-<strong>of</strong>f designed to optimize the<br />
sensitivity and specificity to detect animals with antibodies. The<br />
use <strong>of</strong> antibody titers on a continuous scale can improve the<br />
amount <strong>of</strong> diagnostic information obtained by this technique. The<br />
ELISA evaluated in this study, as is the case <strong>of</strong> previous studies<br />
with different ELISAs 3 could allow a more accurate estimate <strong>of</strong><br />
the situation <strong>of</strong> the animal, in this case related to the<br />
bacteriological “status”.<br />
References<br />
1. Kennedy, DJ, Benedictus, G (2001). Control <strong>of</strong> Mycobacterium avium<br />
subsp. paratuberculosis infection in agricultural species. Scientific and<br />
Technical Reviews, 20, 151-79.<br />
2. World Organization for Animal Health (OIE) (2008). Manual <strong>of</strong> diagnostic<br />
test & vaccines for terrestrial animals, Chapter 2.1.11.<br />
3. Collins, MT (2002). Interpretation <strong>of</strong> a commercial bovine<br />
paratuberculosis enzyme-linked immunosorbent assay by using likelihood<br />
ratios. Clinical and Diagnostic Laboratory Immunology, 9, 1367-71.