OF THE ROGER N. CLARK
OF THE ROGER N. CLARK
OF THE ROGER N. CLARK
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
VISUAL ASTRONOMY <strong>OF</strong> <strong>THE</strong> DEEP SKY<br />
<strong>THE</strong> EYE AND <strong>THE</strong> 'TELESCOPE<br />
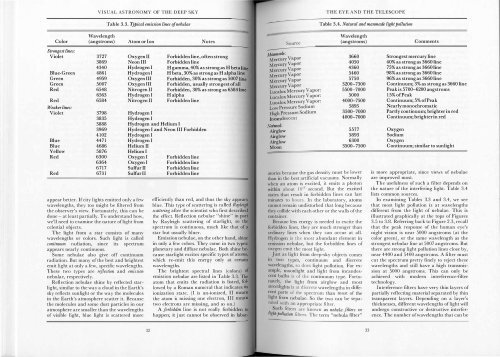
Table 3.3. Typical emission lines ofnebulae<br />
--<br />
Table 3.4. Natural and manmade light pollution<br />
Color<br />
Wavelength<br />
(angstroms) Atom or lon Notes<br />
Strongest lines:<br />
Violet 3727 Oxygen 11 Forbidden line, often strong<br />
3869 Neon III Forbidden line<br />
4340 Hydrogen I H gamma, 40% as strong as H beta line<br />
Blue-Green 4861 Hydrogen I H beta, 30% as strong as H alpha line<br />
Green<br />
Green<br />
Red<br />
4959<br />
5007<br />
6548<br />
Oxygen III<br />
Oxygen III<br />
Nitrogen 11<br />
Forbidden, 30% as strong as 5007 line<br />
Forbidden, usually strongest of all<br />
Forbidden, 30% as strong as 6584 line<br />
6563 Hydrogen I Halpha<br />
Red<br />
6584 Nitrogen 11 Forbidden line<br />
Weaker lines:<br />
Violet 3798<br />
3835<br />
3888<br />
3969<br />
Blue<br />
Blue<br />
Yellow<br />
Red<br />
Red<br />
4102<br />
4471<br />
4686<br />
5876<br />
6300<br />
6364<br />
6717<br />
6731<br />
Hydrogen I<br />
Hydrogen I<br />
Hydrogen and Helium I<br />
Hydrogen I and Neon III Forbidden<br />
Hydrogen I<br />
Hydrogen I<br />
Helium 11<br />
Helium I<br />
Oxygen I<br />
Oxygen I<br />
Sulfur 11<br />
Sulfur 11<br />
appear better. If city lights emitted only a few<br />
wavelengths, they too might be filtered from<br />
the observer's view. Fortunately, this can be<br />
done - at least partially. To understand how,<br />
we'll need to examine the nature of light from<br />
celestial objects.<br />
The light from a star consists of many<br />
wavelengths or colors. Such light is called<br />
continuum radiation, since its spectrum<br />
appears nearly continuous.<br />
Some nebulae also give off continuum<br />
radiation. But many of the best and brightest<br />
emit light at only a few, specific wavelengths.<br />
These two types are reflection and emission<br />
nebulae, respectively.<br />
Reflection nebulae shine by reflected starlight,<br />
similar to the way a cloud in the Earth's<br />
sky reflects sunlight or the way the molecules<br />
in the Earth's atmosphere scatter it. Because<br />
the molecules and some dust particles in our<br />
atmosphere are smaller than the wavelengths<br />
of visible light, blue light is scattered more<br />
Forbidden line<br />
Forbidden line<br />
Forbidden line<br />
Forbidden line<br />
efficiently than red, and thus the sky appears<br />
blue. This type of scattering is called Rayleigh<br />
scattering after the scientist who first described<br />
the effect. Reflection nebulae "shine" in part<br />
by Rayleigh scattering of starlight, so the<br />
spectrum is continuous, much like that of a<br />
star but usually bluer.<br />
Emission nebulae, on the other hand, shine<br />
in only a few colors. They come in two types:<br />
planetary and diffuse nebulae. Both shine because<br />
starlight excites specific types of atoms,<br />
which re-emit this energy only at certain<br />
wavelengths.<br />
The brightest spectral lines (colors)<br />
emission nebulae are listed in Table 3.3. The<br />
atom that emits the radiation is listed, fo l<br />
lowed by a Roman numeral that indicates its<br />
ionization state. (I is un-ionized, II means<br />
the atom is missing one electron, III means<br />
two electrons are missing, and so on.)<br />
A fo rbidden line is not really fo rbidden to<br />
happen; it just cannot be observed in labor-<br />
=-<br />
-<br />
Manmade:<br />
Source<br />
Mercury Vapor<br />
Wavelength<br />
(angstroms)<br />
3660<br />
Mercury Vapor 4050<br />
Mercury Vapor 4360<br />
Mercury Vapor 5460<br />
5750<br />
Mercury Vapor<br />
Mercury Vapor 3200-7300<br />
Lucalox Mercury Vapor: 5500-7000<br />
Lucalox Mercury Vapor:<br />
5000<br />
Lucalox Mercury Vapor: 4000-7500<br />
Low Pressure Sodium<br />
5893<br />
High Pressure Sodium 3500-7000<br />
Incandescent 4000-7000<br />
Natural:<br />
Airglow 5577<br />
Airglow 5893<br />
Airglow<br />
6300<br />
Moon 3500-7500<br />
atories because the gas density must be lower<br />
than in the best artificial vacuums. Normally<br />
when an atom is excited, it emits a photon<br />
within about 10- 8 second. But the excited<br />
states that result in fo rbidden lines can last<br />
minutes to hours. In the laboratory, atoms<br />
cannot remain undisturbed that long because<br />
they collide with each other or the walls of the<br />
container.<br />
Because less energy is needed to excite the<br />
fo rbidden lines, they are much stronger than<br />
ordinary lines when they can occur at all.<br />
Hydrogen is the most abundant element in<br />
emission nebulae, but the forbidden lines of<br />
oxygen emit the most light.<br />
Just as light from deep-sky objects comes<br />
in two types, continuum and discrete<br />
wavelengths, so does light pollution. For example,<br />
moonlight and light from incandescent<br />
bulbs is of the continuum type. Fortunately,<br />
the light from airglow and most<br />
streetlights is at discrete wavelengths in different<br />
parts of the spectrum than most of the<br />
light from nebulae. So the two can be separated<br />
with an appropriate filter.<br />
Such filters are known as nebula filters or<br />
.<br />
bght-pollution filters. The term "nebula filter"<br />
Comments<br />
Strongest mercury line<br />
40% as strong as 3660 line<br />
75% as strong as 3660 line<br />
98% as strong as 3660 line<br />
96% as strong as 3660 line<br />
Continuum; 3% as strong as 3660 line<br />
Peak is 5700-6200 angstroms<br />
15% of Peak<br />
Continuum; 5% of Peak<br />
N early monochromatic<br />
Partly continuum; brighter in red<br />
Continuum; brighter in red<br />
Oxygen<br />
Sodium<br />
Oxygen<br />
Continuum; similar to sunlight<br />
is more appropriate, Since VIews of nebulae<br />
are improved most.<br />
The usefulness of such a filter depends on<br />
the nature of the interfering light. Table 3.4<br />
lists common sources.<br />
In examining Tables 3.3 and 3.4, we see<br />
that most light pollution is at wavelengths<br />
different from the light of nebulae. This is<br />
illustrated graphically at the tops of Figures<br />
3.5 to 3.8. Referring back to Figure 2.3, recall<br />
that the peak response of the human eye's<br />
night vision is near 5000 angstroms (at the<br />
color green), or the same wavelength as the<br />
strongest nebular line at 5007 angstroms. But<br />
there are strong light pollution lines close by,<br />
near 4400 and 5400 angstroms. A filter must<br />
cut the spectrum pretty finely to reject these<br />
wavelengths and still have a high transmission<br />
at 5000 angstroms. This can only be<br />
achieved with modern interference-filter<br />
technology.<br />
Interference filters have very thin layers of<br />
partially reflecting material separated by thin<br />
transparent layers. Depending on a layer's<br />
thicknesses, different wavelengths of light will<br />
undergo constructive or destructive interference.<br />
The number of wavelengths that can be<br />
32<br />
33