ANNUAL REPORT 2012 - TiGenix
ANNUAL REPORT 2012 - TiGenix
ANNUAL REPORT 2012 - TiGenix
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
place a huge burden on not only individuals<br />
but also their health care providers.<br />
Clinical development<br />
<strong>TiGenix</strong> conducted a Phase I clinical trial in<br />
Spain. 10 healthy volunteers, 5 males and 5<br />
females, have been studied in two cohorts<br />
with two different doses. The objective<br />
of this trial was to confirm the feasibility<br />
and the tolerability of the intralymphatic<br />
injection of Cx621. The study consisted of two<br />
intralymphatic administrations one week<br />
apart of Cx621 into the two inguinal nodes,<br />
with a safety follow-up of 21 days after each<br />
administration. In each of the cohorts, two<br />
subjects received placebo (HTS) and the<br />
others Cx621.<br />
Results of this study confirmed that the<br />
intralymphatic administration was possible in<br />
every of the 10 subjects, with no incidences<br />
during or after the administrations, and<br />
with no technical difficulties. There were<br />
13 adverse events in 7 subjects, all of them<br />
mild except one moderate, and none of<br />
them related to the study medication. All<br />
biological parameters and all exploratory<br />
tests were between normal ranges<br />
throughout the study in every case. An<br />
increase in the surface of lymph nodes was<br />
noted by ultrasound imaging of the inguinal<br />
area, more evident in the active group<br />
receiving Cx621 than in the placebo one,<br />
but with no clinical relevance. No significant<br />
changes were observed in the distribution<br />
of cell subsets in the blood of the volunteers,<br />
and no evident changes occurred in the<br />
early activation markers.<br />
In conclusion, the feasibility of the<br />
intralymphatic administration and the<br />
tolerability were confirmed.<br />
6.5.2. Indications and target markets<br />
Based on the competitive advantages<br />
of the eASC anti-inflammatory mode of<br />
action and the choice for an allogeneic<br />
approach, <strong>TiGenix</strong> aims to exploit the<br />
immunomodulatory capacity of eASCs<br />
pursuing the delivery of the cells via the<br />
most appropriate route of administration<br />
according to the indication targeted.<br />
These different routes of administration rely<br />
on either systemic or local administration.<br />
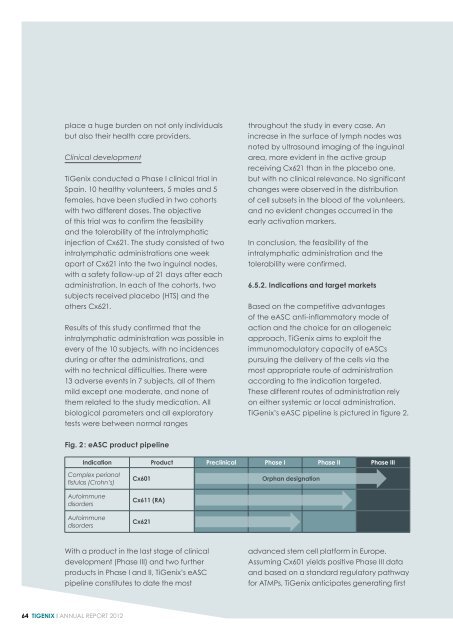
<strong>TiGenix</strong>’s eASC pipeline is pictured in figure 2.<br />
Fig. 2 : eASC product pipeline<br />
Indication Product Preclinical Phase I Phase II Phase III<br />
Complex perianal<br />
fistulas (Crohn’s)<br />
Autoimmune<br />
disorders<br />
Autoimmune<br />
disorders<br />
Cx601<br />
Cx611 (RA)<br />
Cx621<br />
Orphan designation<br />
With a product in the last stage of clinical<br />
development (Phase III) and two further<br />
products in Phase I and II, <strong>TiGenix</strong>’s eASC<br />
pipeline constitutes to date the most<br />
advanced stem cell platform in Europe.<br />
Assuming Cx601 yields positive Phase III data<br />
and based on a standard regulatory pathway<br />
for ATMPs, <strong>TiGenix</strong> anticipates generating first<br />
64 <strong>TiGenix</strong> I annual report <strong>2012</strong>