European Journal of Medical Research - Deutsche AIDS ...
European Journal of Medical Research - Deutsche AIDS ...
European Journal of Medical Research - Deutsche AIDS ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
112 EUROPEAN JOURNAL OF MEDICAL RESEARCH<br />
June 27, 2007<br />
(23/45) at week 12, the rate <strong>of</strong> those being dissatisfied or very<br />
dissatisfied was 26% (11/42) and 20% (9/45), respectively.<br />
Conclusions: Experience within the program suggests that<br />
improvement in satisfaction <strong>of</strong> enfuvirtide patients with therapy<br />
may be achieved by a structured training and experience<br />
exchange program. Detailed repeated instruction and support<br />
may lead to increased treatment acceptance and improved adherence.<br />
1. Lalezari J-P et al. New England J <strong>of</strong> Med., Vol 348, 22 2003<br />
2 Foy K et al. J <strong>of</strong> the Assoc. <strong>of</strong> Nurses in Aids Care, Vol 16, 2<br />
2005<br />
D.83 (Vortrag)<br />
Gesundheitstrainings HIV/<strong>AIDS</strong> - a nationwide<br />
structured patient education program for people<br />
living with HIV/<strong>AIDS</strong> in Germany<br />
Klumb S. 1 , Dannecker M. 1 , Gronski H. 1 , Schafberger A. 1<br />
1 Verein Gesundheitstraining HIV/<strong>AIDS</strong> e.V., Berlin, Germany<br />
Background: In 1998 a working group <strong>of</strong> HIV+ patients and<br />
treatment advocates started to develop a structured patient education<br />
program for people living with HIV/<strong>AIDS</strong> in Germany.<br />
From our perspective adherence is a consequence <strong>of</strong><br />
psychosocial factors and processes.<br />
From July 2005 on eight patient trainings have been conducted,<br />
61 patients were included. Trainings have been conducted<br />
in groups. Group size varied (mean 10.1, range 5 - 12).<br />
Mean age was 41.2 years. 15.1 % <strong>of</strong> participants were female<br />
84.9 % male.<br />
Two types <strong>of</strong> research have been conducted resp. are ongoing:<br />
an evaluation <strong>of</strong> the curricula and the qualification <strong>of</strong><br />
trainers (completed), and an evaluation <strong>of</strong> health economic<br />
consequences and long lasting effectiveness (still ongoing).<br />
Methods: The research utilized questionnaires several times<br />
during the program and 6 month after the last training session.<br />
In addition two group discussions leaded by the researchers<br />
were conducted as qualitative part.<br />
The second part <strong>of</strong> the research used two questionnaires<br />
(MOS-HIV and HADS) and collected “hard data” via self report<br />
and patients’ health insurance companies. Questionnaires<br />
and data collection was carried out after inclusion but before<br />
the first training session and 12 month after the last training<br />
session.<br />
Results: 41 patients were included in this OT-style analysis<br />
(LOF = 2, 18 did not fill out the last or any other questionnaire).<br />
Six month after the last training session 53.7 % (n =<br />
22) stated, that the patient education program has been very<br />
helpful and 41.5 % (n = 17) that it has been helpful in terms<br />
<strong>of</strong> living with HIV. Only 2.4 % respectively stated the intervention<br />
has been somewhat (n = 1) or not helpful (n = 1).<br />
Table 1<br />
The 95.2 % (n = 39) patients gave the following key topics<br />
as most valuable:<br />
1. exchange <strong>of</strong> experience (n= 21)<br />
2. develop a new (different) view <strong>of</strong> living with HIV (more<br />
self confidence/ less anxiety / improved QoL) (n= 16)<br />
3. information / diet / sport / coping with stress (n = 12)<br />
4. HAART / management <strong>of</strong> side effects / therapeutic options<br />
and strategies (n = 11)<br />
5. due to knowledge and training a more specific / focussed<br />
decision-making (n = 7)<br />
More detailed data and the results <strong>of</strong> ongoing evaluation <strong>of</strong><br />
“hard data” will be provided.<br />
D.84 (Poster)<br />
Health related quality <strong>of</strong> life changes in<br />
antiretroviral naïve HIV-infected patients on<br />
Atazanavir based regimens: Week 48 results from<br />
AI424-089<br />
Iloeje U. 1 , Kastango K. 1 , Malan N. 2 , David N. 3 ,<br />
Krantz E. 4 , Reeb I. 5 , Nakonz T. 5 , Matthew M. 1 ,<br />
Frederick D. 6 , Hammond J. 6<br />
1 Bristol-Myers Squibb, Pharmaceutical <strong>Research</strong> Institute,<br />
Wallingford, United States <strong>of</strong> America, 2 Triple M <strong>Research</strong>,<br />
Port Elizabeth, South Africa, 3 Brooklyn <strong>Medical</strong> <strong>Research</strong><br />
Center, Cape Town, South Africa, 4 Quinta-<strong>Research</strong>,<br />
Bloemfontein, South Africa, 5 Bristol-Myers Squibb<br />
GmbH&Co.KGaA, Munich, Germany, 6 Bristol-Myers Squibb,<br />
Wallingford, United States <strong>of</strong> America<br />
Background: HRQoL declines in HIV-infected patients in<br />
the absence <strong>of</strong> effective therapy or with older more symptomatic<br />
ARV regimens. Atazanavir is potent and well tolerated<br />
as a once daily protease inhibitor within a HAART regimen.<br />
Data on HRQoL in treatment naïve patients on ATV/r or ATV<br />
regimens are limited.<br />
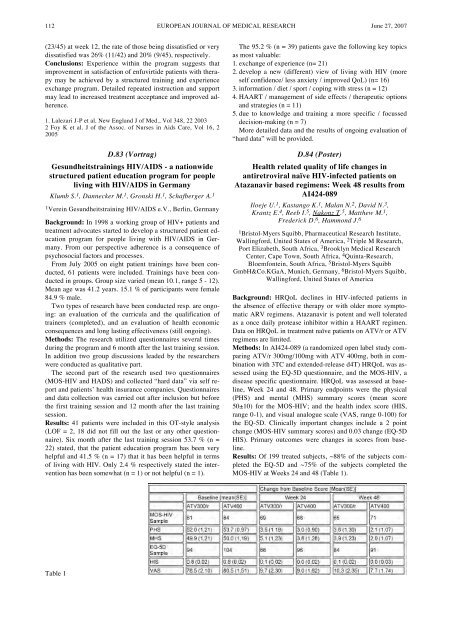
Methods: In AI424-089 (a randomized open label study comparing<br />
ATV/r 300mg/100mg with ATV 400mg, both in combination<br />
with 3TC and extended-release d4T) HRQoL was assessed<br />
using the EQ-5D questionnaire, and the MOS-HIV, a<br />
disease specific questionnaire. HRQoL was assessed at baseline,<br />
Week 24 and 48. Primary endpoints were the physical<br />
(PHS) and mental (MHS) summary scores (mean score<br />
50±10) for the MOS-HIV; and the health index score (HIS,<br />
range 0-1), and visual analogue scale (VAS, range 0-100) for<br />
the EQ-5D. Clinically important changes include a 2 point<br />
change (MOS-HIV summary scores) and 0.03 change (EQ-5D<br />
HIS). Primary outcomes were changes in scores from baseline.<br />
Results: Of 199 treated subjects, ~88% <strong>of</strong> the subjects completed<br />
the EQ-5D and ~75% <strong>of</strong> the subjects completed the<br />
MOS-HIV at Weeks 24 and 48 (Table 1).