Standard X-ray Diffraction Powder Patterns
Standard X-ray Diffraction Powder Patterns
Standard X-ray Diffraction Powder Patterns
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
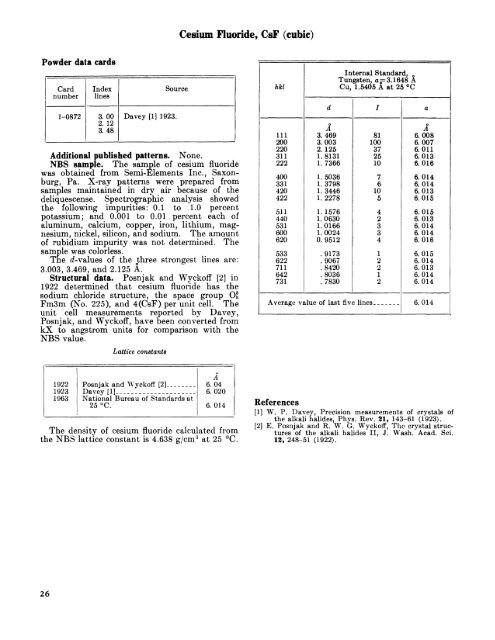
Cesium Fluoride, CsF (cubic)<strong>Powder</strong> data cardsCardnumber1-0872Indexlines3. 002. 123. 48Davey [1] 1923.SourceAdditional published patterns. None.NBS sample. The sample of cesium fluoridewas obtained from Semi-Elements Inc., Saxonburg,Pa. X-<strong>ray</strong> patterns were prepared fromsamples maintained in dry air because of thedeliquescense. Spectrpgraphic analysis showedthe following impurities: 0.1 to 1.0 percentpotassium; and 0.001 to 0.01 percent each ofaluminum, calcium, copper, iron, lithium, magnesium, nickel, silicon, and sodium. The amountof rubidium impurity was not determined. Thesample was colorless.The d-values of the three strongest lines are:3.003, 3.469, and 2.125 A.Structural data. Posnjak and Wyckoff [2] in1922 determined that cesium fluoride has thesodium chloride structure, the space group O£Fm3m (No. 225), and 4(CsF) per unit cell. Theunit cell measurements reported by Davey,Posnjak, and Wyckoff, have been converted fromkX to angstrom units for comparison with theNBS value.Lattice constantshkl111200220311222400331420422511440531600620533622711642731dA3.4693.0032. 1251. 81311. 73661. 50361. 37981. 34461. 22781. 15761. 06301.01661. 00240. 9512.9173.9067.8420.8036. 7830Internal <strong>Standard</strong>,Tungsten, a = 3. 1648 ACu, 1.5405 A at 25 °CAverage value of last five> lines _ . _I81100372510761054233412212a0A6.0086.0076.0116.0136.0166.0146.0146.0136.0156.0156.0136.0146.0146.0166.0156.0146.0136.0146.0146.014192219231963Posnjak and Wyckoff [2] _ ___ _Davey [1]_ ______ _._National Bureau of <strong>Standard</strong>s at25 °C.A6. 046. 0206. 014The density of cesium fluoride calculated fromthe NBS lattice constant is 4.638 g/cm 3 at 25 °C.References[1] W. P. Davev, Precision measurements of crystals ofthe alkali halides, Phys. Rev. 21, 143-61 (1923).[2] E. Posnjak and R. W. G. Wyckoff, The crystal structures of the alkali halides II, J. Wash. Acad. Sci.13, 248-51 (1922).26