Cultivating Excellence Fact Book 2015–2016
agrium_fb_2015-2016_web
agrium_fb_2015-2016_web
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
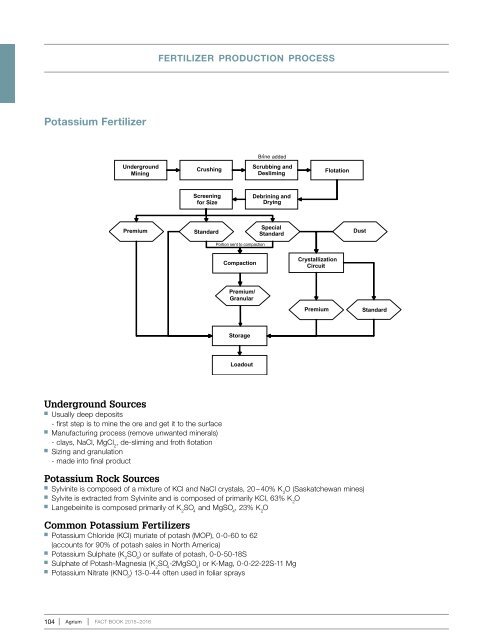
fertilizer production process<br />
Potassium Fertilizer<br />
Underground Sources<br />
n<br />
Usually deep deposits<br />
- first step is to mine the ore and get it to the surface<br />
n<br />
Manufacturing process (remove unwanted minerals)<br />
- clays, NaCl, MgCl 2<br />
, de-sliming and froth flotation<br />
n<br />
Sizing and granulation<br />
- made into final product<br />
Potassium Rock Sources<br />
n<br />
Sylvinite is composed of a mixture of KCl and NaCl crystals, 20 – 40% K 2<br />
O (Saskatchewan mines)<br />
n<br />
Sylvite is extracted from Sylvinite and is composed of primarily KCl, 63% K 2<br />
O<br />
n<br />
Langebeinite is composed primarily of K 2<br />
SO 4<br />
and MgSO 4<br />
, 23% K 2<br />
O<br />
Common Potassium Fertilizers<br />
n<br />
Potassium Chloride (KCl) muriate of potash (MOP), 0-0-60 to 62<br />
(accounts for 90% of potash sales in North America)<br />
n<br />
Potassium Sulphate (K 2<br />
SO 4<br />
) or sulfate of potash, 0-0-50-18S<br />
n<br />
Sulphate of Potash-Magnesia (K 2<br />
SO 4<br />
-2MgSO 4<br />
) or K-Mag, 0-0-22-22S-11 Mg<br />
n<br />
Potassium Nitrate (KNO 3<br />
) 13-0-44 often used in foliar sprays<br />
104 Agrium FACT BOOK <strong>2015–2016</strong>