Scientific Theme: Advanced Modeling and Observing Systems
Scientific Theme: Advanced Modeling and Observing Systems
Scientific Theme: Advanced Modeling and Observing Systems
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Scientific</strong> <strong>Theme</strong>: Regional Processes<br />
study appeared in Atmospheric Chemistry <strong>and</strong> Physics in January 2007, <strong>and</strong> results from NEAQS 2004 are currently<br />
in press in the Journal of Geophysical Research. Results from TexAQS 2006 <strong>and</strong> Erie 2007 are anticipated in the<br />
coming year.<br />
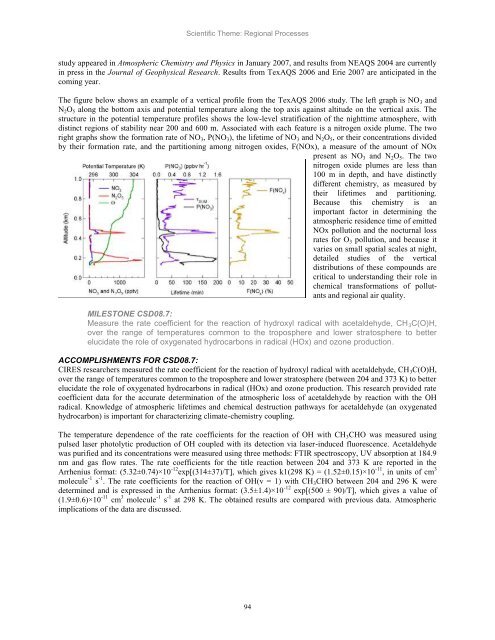
The figure below shows an example of a vertical profile from the TexAQS 2006 study. The left graph is NO3 <strong>and</strong><br />
N2O5 along the bottom axis <strong>and</strong> potential temperature along the top axis against altitude on the vertical axis. The<br />
structure in the potential temperature profiles shows the low-level stratification of the nighttime atmosphere, with<br />
distinct regions of stability near 200 <strong>and</strong> 600 m. Associated with each feature is a nitrogen oxide plume. The two<br />
right graphs show the formation rate of NO3, P(NO3), the lifetime of NO3 <strong>and</strong> N2O5, or their concentrations divided<br />
by their formation rate, <strong>and</strong> the partitioning among nitrogen oxides, F(NOx), a measure of the amount of NOx<br />
present as NO3 <strong>and</strong> N2O5. The two<br />
nitrogen oxide plumes are less than<br />
100 m in depth, <strong>and</strong> have distinctly<br />
different chemistry, as measured by<br />
their lifetimes <strong>and</strong> partitioning.<br />
Because this chemistry is an<br />
important factor in determining the<br />
atmospheric residence time of emitted<br />
NOx pollution <strong>and</strong> the nocturnal loss<br />
rates for O3 pollution, <strong>and</strong> because it<br />
varies on small spatial scales at night,<br />
detailed studies of the vertical<br />
distributions of these compounds are<br />
critical to underst<strong>and</strong>ing their role in<br />
chemical transformations of pollutants<br />
<strong>and</strong> regional air quality.<br />
MILESTONE CSD08.7:<br />
Measure the rate coefficient for the reaction of hydroxyl radical with acetaldehyde, CH3C(O)H,<br />
over the range of temperatures common to the troposphere <strong>and</strong> lower stratosphere to better<br />
elucidate the role of oxygenated hydrocarbons in radical (HOx) <strong>and</strong> ozone production.<br />
ACCOMPLISHMENTS FOR CSD08.7:<br />
CIRES researchers measured the rate coefficient for the reaction of hydroxyl radical with acetaldehyde, CH3C(O)H,<br />
over the range of temperatures common to the troposphere <strong>and</strong> lower stratosphere (between 204 <strong>and</strong> 373 K) to better<br />
elucidate the role of oxygenated hydrocarbons in radical (HOx) <strong>and</strong> ozone production. This research provided rate<br />
coefficient data for the accurate determination of the atmospheric loss of acetaldehyde by reaction with the OH<br />
radical. Knowledge of atmospheric lifetimes <strong>and</strong> chemical destruction pathways for acetaldehyde (an oxygenated<br />
hydrocarbon) is important for characterizing climate-chemistry coupling.<br />
The temperature dependence of the rate coefficients for the reaction of OH with CH3CHO was measured using<br />
pulsed laser photolytic production of OH coupled with its detection via laser-induced fluorescence. Acetaldehyde<br />
was purified <strong>and</strong> its concentrations were measured using three methods: FTIR spectroscopy, UV absorption at 184.9<br />
nm <strong>and</strong> gas flow rates. The rate coefficients for the title reaction between 204 <strong>and</strong> 373 K are reported in the<br />
Arrhenius format: (5.32±0.74)×10 -12 exp[(314±37)/T], which gives k1(298 K) = (1.52±0.15)×10 -11 , in units of cm 3<br />
molecule -1 s -1 . The rate coefficients for the reaction of OH(v = 1) with CH3CHO between 204 <strong>and</strong> 296 K were<br />
determined <strong>and</strong> is expressed in the Arrhenius format: (3.5±1.4)×10 -12 exp[(500 ± 90)/T], which gives a value of<br />
(1.9±0.6)×10 -11 cm 3 molecule -1 s -1 at 298 K. The obtained results are compared with previous data. Atmospheric<br />
implications of the data are discussed.<br />
94