THE ROLE OF THE
THE ROLE OF THE
THE ROLE OF THE
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
88 The Role of the Chemist in Automotive Design<br />
Unlike inert fillers that have little effect on physical properties, reinforcing fillers<br />
such as carbon black and Teflon add to the mechanical properties of the polymer [6].<br />
Both of the polymers for rotating dynamic seals examined here contain carbon black<br />
as a reinforcing filler. Teflon is added to polyetheretherketone to increase the lubricity<br />
properties of the polymer. Ninety percent of carbon black is obtained through<br />
furnace black, which involves the combustion of thermal cracking of hydrocarbons<br />
[7]. This reaction takes place at 1,200–1,400°C:<br />
–CH 2– → C + H 2<br />
–CH 2– + 1½ O 2 → CO 2 + H 2O (6.1)<br />
Another benefit of carbon black may be the potential for protection against oxidation.<br />
Due to the higher temperatures over an extended period of time, thermal<br />
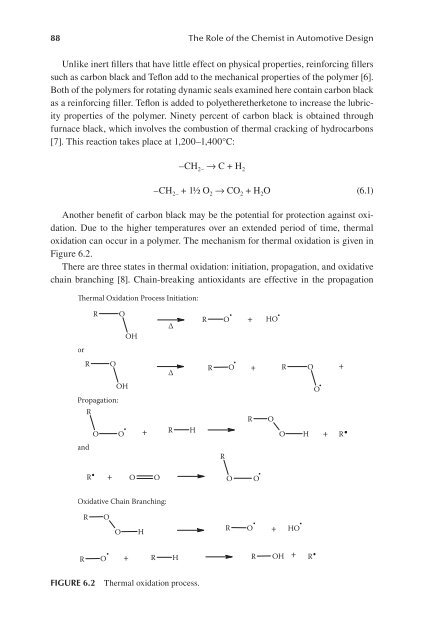
oxidation can occur in a polymer. The mechanism for thermal oxidation is given in<br />
Figure 6.2.<br />
There are three states in thermal oxidation: initiation, propagation, and oxidative<br />
chain branching [8]. Chain-breaking antioxidants are effective in the propagation<br />
Thermal Oxidation Process Initiation:<br />
or<br />
R O<br />
R O<br />
Propagation:<br />
R<br />
and<br />
OH<br />
OH<br />
∆<br />
∆<br />
O O + R H<br />
R + O O<br />
Oxidative Chain Branching:<br />
R O<br />
O<br />
H<br />
R O + HO<br />
R O + R O<br />
R<br />
O O<br />
R O<br />
O<br />
R O + HO<br />
R O + R H<br />
R OH + R<br />
FIgure 6.2 Thermal oxidation process.<br />
H<br />
O<br />
+<br />
+<br />
R