THE ROLE OF THE
THE ROLE OF THE
THE ROLE OF THE
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
152 The Role of the Chemist in Automotive Design<br />
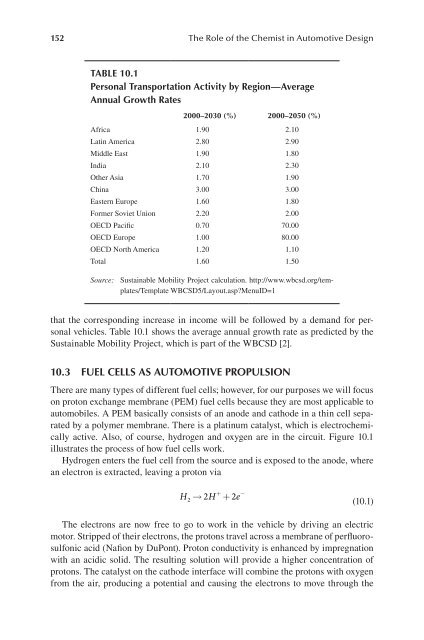
table 10.1<br />
Personal transportation activity by region—average<br />
annual growth rates<br />
2000–2030 (%) 2000–2050 (%)<br />
Africa 1.90 2.10<br />
Latin America 2.80 2.90<br />
Middle East 1.90 1.80<br />
India 2.10 2.30<br />
Other Asia 1.70 1.90<br />
China 3.00 3.00<br />
Eastern Europe 1.60 1.80<br />
Former Soviet Union 2.20 2.00<br />
OECD Pacific 0.70 70.00<br />
OECD Europe 1.00 80.00<br />
OECD North America 1.20 1.10<br />
Total 1.60 1.50<br />
Source: Sustainable Mobility Project calculation. http://www.wbcsd.org/templates/Template<br />
WBCSD5/Layout.asp?MenuID=1<br />
that the corresponding increase in income will be followed by a demand for personal<br />
vehicles. Table 10.1 shows the average annual growth rate as predicted by the<br />
Sustainable Mobility Project, which is part of the WBCSD [2].<br />
10.3 Fuel cells as automotIve ProPulsIon<br />
There are many types of different fuel cells; however, for our purposes we will focus<br />
on proton exchange membrane (PEM) fuel cells because they are most applicable to<br />
automobiles. A PEM basically consists of an anode and cathode in a thin cell separated<br />
by a polymer membrane. There is a platinum catalyst, which is electrochemically<br />
active. Also, of course, hydrogen and oxygen are in the circuit. Figure 10.1<br />
illustrates the process of how fuel cells work.<br />
Hydrogen enters the fuel cell from the source and is exposed to the anode, where<br />
an electron is extracted, leaving a proton via<br />
H2→ 2H + 2e<br />
+ −<br />
(10.1)<br />
The electrons are now free to go to work in the vehicle by driving an electric<br />
motor. Stripped of their electrons, the protons travel across a membrane of perfluorosulfonic<br />
acid (Nafion by DuPont). Proton conductivity is enhanced by impregnation<br />
with an acidic solid. The resulting solution will provide a higher concentration of<br />
protons. The catalyst on the cathode interface will combine the protons with oxygen<br />
from the air, producing a potential and causing the electrons to move through the