THE ROLE OF THE
THE ROLE OF THE
THE ROLE OF THE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Developing Technology 179<br />
Positive<br />
LiCoO 2<br />
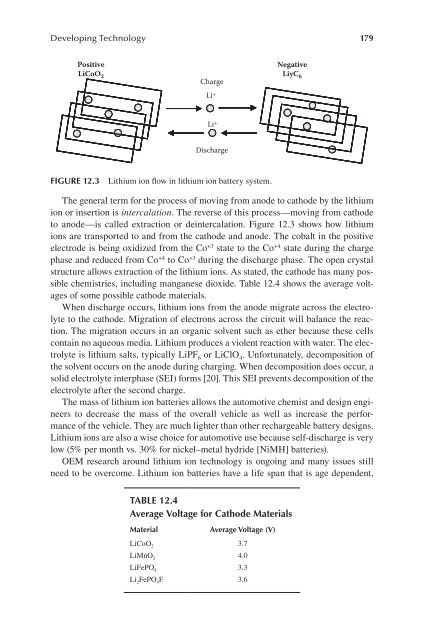
The general term for the process of moving from anode to cathode by the lithium<br />
ion or insertion is intercalation. The reverse of this process—moving from cathode<br />
to anode—is called extraction or deintercalation. Figure 12.3 shows how lithium<br />
ions are transported to and from the cathode and anode. The cobalt in the positive<br />
electrode is being oxidized from the Co +3 state to the Co +4 state during the charge<br />
phase and reduced from Co +4 to Co +3 during the discharge phase. The open crystal<br />
structure allows extraction of the lithium ions. As stated, the cathode has many possible<br />
chemistries, including manganese dioxide. Table 12.4 shows the average voltages<br />
of some possible cathode materials.<br />
When discharge occurs, lithium ions from the anode migrate across the electrolyte<br />
to the cathode. Migration of electrons across the circuit will balance the reaction.<br />
The migration occurs in an organic solvent such as ether because these cells<br />
contain no aqueous media. Lithium produces a violent reaction with water. The electrolyte<br />
is lithium salts, typically LiPF 6 or LiClO 4. Unfortunately, decomposition of<br />
the solvent occurs on the anode during charging. When decomposition does occur, a<br />
solid electrolyte interphase (SEI) forms [20]. This SEI prevents decomposition of the<br />
electrolyte after the second charge.<br />
The mass of lithium ion batteries allows the automotive chemist and design engineers<br />
to decrease the mass of the overall vehicle as well as increase the performance<br />
of the vehicle. They are much lighter than other rechargeable battery designs.<br />
Lithium ions are also a wise choice for automotive use because self-discharge is very<br />
low (5% per month vs. 30% for nickel–metal hydride [NiMH] batteries).<br />
OEM research around lithium ion technology is ongoing and many issues still<br />
need to be overcome. Lithium ion batteries have a life span that is age dependent,<br />
table 12.4<br />
average voltage for cathode materials<br />
material average voltage (v)<br />
LiCoO 2<br />
LiMnO 2<br />
LiFePO 4<br />
Charge<br />
Li +<br />
Li +<br />
Discharge<br />
FIgure 12.3 Lithium ion flow in lithium ion battery system.<br />
3.7<br />
4.0<br />
3.3<br />
Li 2FePO 4F 3.6<br />
Negative<br />
LiyC 6