THE ROLE OF THE
THE ROLE OF THE
THE ROLE OF THE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Engineering and Structural Polymers 89<br />
R<br />
Stabilization by labile-hydrogen donating antioxidant (HA)<br />
O O +<br />
stage of this process. They react with the radicals that propagate oxidation, thus<br />
reducing the hydrogen donating antioxidant (HA) shown in Figure 6.3.<br />
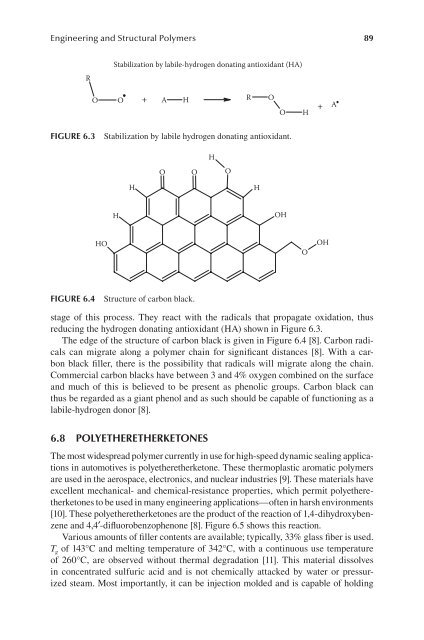
The edge of the structure of carbon black is given in Figure 6.4 [8]. Carbon radicals<br />
can migrate along a polymer chain for significant distances [8]. With a carbon<br />
black filler, there is the possibility that radicals will migrate along the chain.<br />
Commercial carbon blacks have between 3 and 4% oxygen combined on the surface<br />
and much of this is believed to be present as phenolic groups. Carbon black can<br />
thus be regarded as a giant phenol and as such should be capable of functioning as a<br />
labile-hydrogen donor [8].<br />
6.8 PolyetHeretHerKetones<br />
A<br />
H<br />
R O<br />
FIgure 6.3 Stabilization by labile hydrogen donating antioxidant.<br />
HO<br />
H<br />
FIgure 6.4 Structure of carbon black.<br />
H<br />
O<br />
O<br />
H<br />
The most widespread polymer currently in use for high-speed dynamic sealing applications<br />
in automotives is polyetheretherketone. These thermoplastic aromatic polymers<br />
are used in the aerospace, electronics, and nuclear industries [9]. These materials have<br />
excellent mechanical- and chemical-resistance properties, which permit polyetheretherketones<br />
to be used in many engineering applications—often in harsh environments<br />
[10]. These polyetheretherketones are the product of the reaction of 1,4-dihydroxybenzene<br />
and 4,4′-difluorobenzophenone [8]. Figure 6.5 shows this reaction.<br />
Various amounts of filler contents are available; typically, 33% glass fiber is used.<br />
T g of 143°C and melting temperature of 342°C, with a continuous use temperature<br />
of 260°C, are observed without thermal degradation [11]. This material dissolves<br />
in concentrated sulfuric acid and is not chemically attacked by water or pressurized<br />
steam. Most importantly, it can be injection molded and is capable of holding<br />
O<br />
H<br />
O<br />
OH<br />
H<br />
O<br />
+<br />
OH<br />
A