THE ROLE OF THE
THE ROLE OF THE
THE ROLE OF THE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
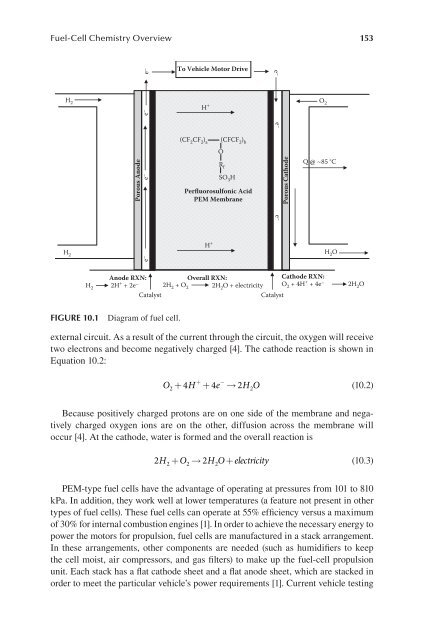
Fuel-Cell Chemistry Overview 153<br />
H 2<br />
H 2<br />
H 2<br />
e –<br />
Porous Anode<br />
e –<br />
e – e –<br />
To Vehicle Motor Drive<br />
H +<br />
(CF 2 CF 2 ) a<br />
H +<br />
external circuit. As a result of the current through the circuit, the oxygen will receive<br />
two electrons and become negatively charged [4]. The cathode reaction is shown in<br />
Equation 10.2:<br />
+ −<br />
O + 4H + 4e → 2H<br />
O<br />
2 2<br />
(10.2)<br />
Because positively charged protons are on one side of the membrane and negatively<br />
charged oxygen ions are on the other, diffusion across the membrane will<br />
occur [4]. At the cathode, water is formed and the overall reaction is<br />
2H + O → 2HO+<br />
electricity<br />
(10.3)<br />
2 2 2<br />
(CFCF 2 ) b<br />
PEM-type fuel cells have the advantage of operating at pressures from 101 to 810<br />
kPa. In addition, they work well at lower temperatures (a feature not present in other<br />
types of fuel cells). These fuel cells can operate at 55% efficiency versus a maximum<br />
of 30% for internal combustion engines [1]. In order to achieve the necessary energy to<br />
power the motors for propulsion, fuel cells are manufactured in a stack arrangement.<br />
In these arrangements, other components are needed (such as humidifiers to keep<br />
the cell moist, air compressors, and gas filters) to make up the fuel-cell propulsion<br />
unit. Each stack has a flat cathode sheet and a flat anode sheet, which are stacked in<br />
order to meet the particular vehicle’s power requirements [1]. Current vehicle testing<br />
O<br />
R f<br />
SO 3 H<br />
Perfluorosulfonic Acid<br />
PEM Membrane<br />
Anode RXN:<br />
2H<br />
Catalyst Catalyst<br />
+ + 2e –<br />
Cathode RXN:<br />
O2 + 4H + + 4e – Overall RXN:<br />
2H2 + O2 2H2O + electricity<br />
2H2O FIgure 10.1 Diagram of fuel cell.<br />
e – e–<br />
Porous Cathode<br />
e –<br />
O 2<br />
Q @ ~85 °C<br />
H 2 O