THE ROLE OF THE
THE ROLE OF THE
THE ROLE OF THE
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Power Train Applications 107<br />
7.15 engIne coolant<br />
Engine coolants used in internal combustion engines can be considered to be<br />
cryoprotectant or colligative agents. Cryoprotectants are basically agents added<br />
to water to reduce the freeze point of a mixture. Colligative agents describe the<br />
behavior of coolant in warmer environments because they have beneficial behavior<br />
on both the freezing and boiling ends of the spectrum. It is these antiboil and<br />
antifreeze elements that the chemist seeks when he or she attempts to design an<br />
engine coolant.<br />
Water, of course, was the first material used as an engine coolant. However, alcohols<br />
such as methanol and glycols were soon utilized as a standard for engine coolant.<br />
In 1926, ethylene glycol became available as “permanent antifreeze.” Ethylene<br />
glycol’s higher boiling points increase the operational range of the coolant.<br />
7.16 metHanol<br />
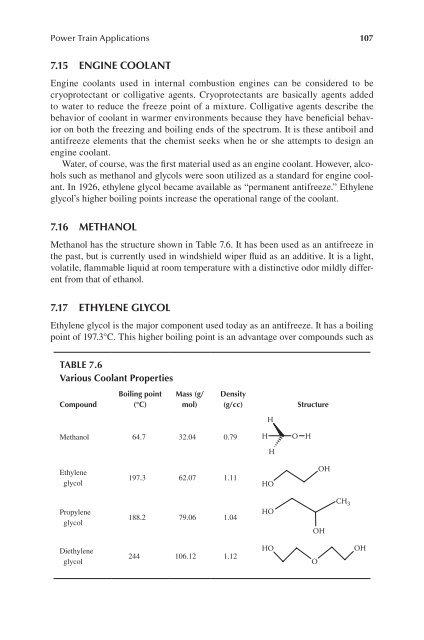
Methanol has the structure shown in Table 7.6. It has been used as an antifreeze in<br />
the past, but is currently used in windshield wiper fluid as an additive. It is a light,<br />
volatile, flammable liquid at room temperature with a distinctive odor mildly different<br />
from that of ethanol.<br />
7.17 etHylene glycol<br />
Ethylene glycol is the major component used today as an antifreeze. It has a boiling<br />
point of 197.3°C. This higher boiling point is an advantage over compounds such as<br />
table 7.6<br />
various coolant Properties<br />
compound<br />
boiling point<br />
(°c)<br />
mass (g/<br />
mol)<br />
Methanol 64.7 32.04 0.79<br />
Ethylene<br />
glycol<br />
Propylene<br />
glycol<br />
Diethylene<br />
glycol<br />
197.3 62.07 1.11<br />
188.2 79.06 1.04<br />
244 106.12 1.12<br />
density<br />
(g/cc) structure<br />
H<br />
H<br />
H<br />
HO<br />
HO<br />
HO<br />
O<br />
H<br />
OH<br />
OH<br />
O<br />
CH 3<br />
OH