THE ROLE OF THE
THE ROLE OF THE
THE ROLE OF THE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
42 The Role of the Chemist in Automotive Design<br />
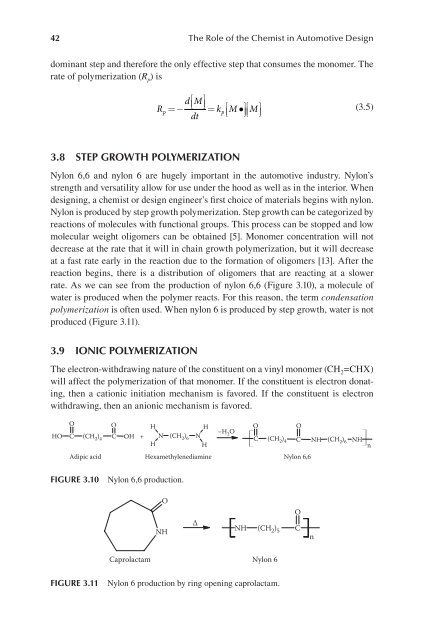
dominant step and therefore the only effective step that consumes the monomer. The<br />
rate of polymerization (R p) is<br />
R<br />
dM ⎡ ⎤<br />
=− ⎣⎢ ⎦⎥<br />
= k ⎡M•⎤M<br />
dt ⎣⎢ ⎦⎥ ⎡ ⎤<br />
⎣⎢ ⎦⎥<br />
p p<br />
3.8 steP growtH PolymerIzatIon<br />
(3.5)<br />
Nylon 6,6 and nylon 6 are hugely important in the automotive industry. Nylon’s<br />
strength and versatility allow for use under the hood as well as in the interior. When<br />
designing, a chemist or design engineer’s first choice of materials begins with nylon.<br />
Nylon is produced by step growth polymerization. Step growth can be categorized by<br />
reactions of molecules with functional groups. This process can be stopped and low<br />
molecular weight oligomers can be obtained [5]. Monomer concentration will not<br />
decrease at the rate that it will in chain growth polymerization, but it will decrease<br />
at a fast rate early in the reaction due to the formation of oligomers [13]. After the<br />
reaction begins, there is a distribution of oligomers that are reacting at a slower<br />
rate. As we can see from the production of nylon 6,6 (Figure 3.10), a molecule of<br />
water is produced when the polymer reacts. For this reason, the term condensation<br />
polymerization is often used. When nylon 6 is produced by step growth, water is not<br />
produced (Figure 3.11).<br />
3.9 IonIc PolymerIzatIon<br />
The electron-withdrawing nature of the constituent on a vinyl monomer (CH 2=CHX)<br />
will affect the polymerization of that monomer. If the constituent is electron donating,<br />
then a cationic initiation mechanism is favored. If the constituent is electron<br />
withdrawing, then an anionic mechanism is favored.<br />
O<br />
O<br />
HO C (CH2 ) 4 C OH +<br />
H<br />
N<br />
H<br />
(CH 2 ) 6<br />
H<br />
N<br />
H<br />
–H 2 O<br />
(CH 2 ) 4<br />
Adipic acid Hexamethylenediamine Nylon 6,6<br />
FIgure 3.10 Nylon 6,6 production.<br />
O<br />
NH<br />
∆<br />
NH<br />
O<br />
C<br />
(CH 2 ) 5<br />
Caprolactam Nylon 6<br />
FIgure 3.11 Nylon 6 production by ring opening caprolactam.<br />
O<br />
C<br />
O<br />
C<br />
NH<br />
n<br />
(CH 2 ) 6<br />
NH<br />
n