Transactions
Transactions
Transactions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
494 TRANSACTIONS OF T H E A.S.M.E. AUGUST, 1941<br />
inhibition, and what molecular-weight acids might give rise to<br />
rusting. I t was found, as might be expected, that the acids of<br />
lower molecular weight, such as formic, acetic, and even propionic,<br />
were rust producers. In fact, more rust was produced by such<br />
acids than by a neutral untreated white oil. Ascending the scale<br />
of acids, complete protection was found through the use of 0.25<br />
per cent caproic acid, having a molecular weight of 116. All acids<br />
of higher molecular weight also give protection. Later experiments<br />
indicated that 0.1 per cent of caproic acid was equally<br />
effective.<br />
Fig. 7 shows a series of ten steel-corrosion specimens, indicating<br />
the relative rusting and rust-inhibiting qualities of 0.25 per cent<br />
of various organic acids in white medicinal oil. All tests were<br />
carried out at 140 F, the oils with 1 per cent water mixture having<br />
been stirred for a total of 16 hr, and left in a quiescent state for a<br />
total of 13 hr. The results are given in Table 2.<br />
T A B L E 2<br />
R ESU L T S OF C O R R O S IO N TESTS O N ST E EL<br />
S P E C IM E N S S H O W N IN F IG . 7<br />
Carbon Molecular<br />
Percentage<br />
atoms in weight of of surface<br />
acid acid rusted<br />
No acid............................................................................................................5<br />
Formic a c id ................................ 1 46 100<br />
Acetic acid .................................. 2 60 100<br />
Propionic a c id ............................ 3 74 50<br />
Butyric a c id ................................ 4 88 20<br />
Valeric a c id ................................ 5 88 2<br />
Caproic a c id ............................... 6 116 0<br />
Caprylic a c id .............................. 8 144 0<br />
Palmitic a c id .............................. 16 256 0<br />
Stearic a c id ................................. 18 284 0<br />
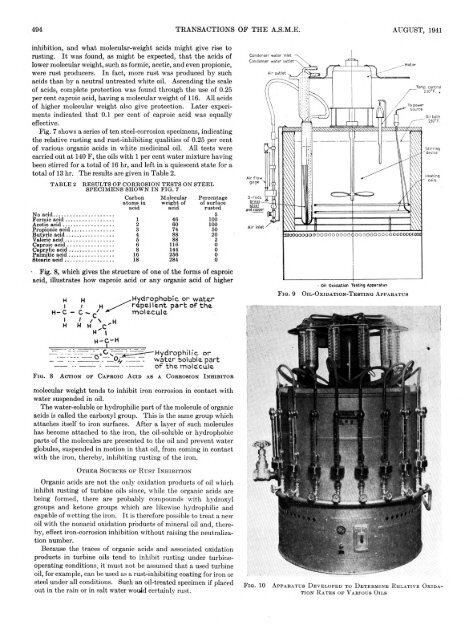
■Fig. 8, which gives the structure of one of the forms of caproic<br />
acid, illustrates how caproic acid or any organic acid of higher<br />
F i g . 8 A c t i o n o p C a p r o i c A c i d a s a C o r r o s i o n I n h i b i t o r<br />
molecular weight tends to inhibit iron corrosion in contact with<br />
water suspended in oil.<br />
The water-soluble or hydrophilic part of the molecule of organic<br />
acids is called the carboxyl group. This is the same group which<br />
attaches itself to iron surfaces. After a layer of such molecules<br />
has become attached to the iron, the oil-soluble or hydrophobic<br />
parts of the molecules are presented to the oil and prevent water<br />
globules, suspended in motion in that oil, from coming in contact<br />
with the iron, thereby, inhibiting rusting of the iron.<br />
O t h e r S o u r c e s o p R u s t I n h i b i t i o n<br />
Organic acids are not the only oxidation products of oil which<br />
inhibit rusting of turbine oils since, while the organic acids are<br />
being formed, there are probably compounds with hydroxyl<br />
groups and ketone groups which are likewise hydrophilic and<br />
capable of wetting the iron. It is therefore possible to treat a new<br />
oil with the nonacid oxidation products of mineral oil and, thereby,<br />
effect iron-corrosion inhibition without raising the neutralization<br />
number.<br />
Because the traces of organic acids and associated oxidation<br />
products in turbine oils tend to inhibit rusting under turbineoperating<br />
conditions, it must not be assumed that a used turbine<br />
oil, for example, can be used as a rust-inhibiting coating for iron or<br />
steel under all conditions. Such an oil-treated specimen if placed<br />
out in the rain or in salt water would certainlv rust.<br />
F i g . 1 0 A p p a r a t u s D e v e l o p e d t o D e t e r m i n e R e l a t i v e O x i d a <br />
t i o n R a t e s o f V a r i o u s O i l s