Beer : Health and Nutrition
Beer : Health and Nutrition
Beer : Health and Nutrition
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The Impact of Alcohol on <strong>Health</strong> 143<br />
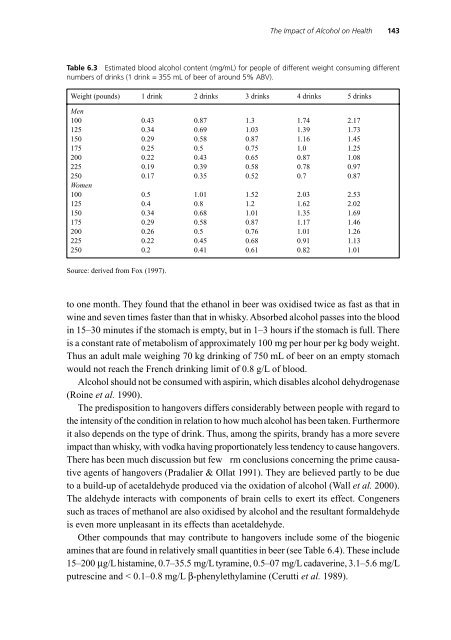
Table 6.3 Estimated blood alcohol content (mg/mL) for people of different weight consuming different<br />
numbers of drinks (1 drink = 355 mL of beer of around 5% ABV).<br />
Weight (pounds) 1 drink 2 drinks 3 drinks 4 drinks 5 drinks<br />
Men<br />
100 0.43 0.87 1.3 1.74 2.17<br />
125 0.34 0.69 1.03 1.39 1.73<br />
150 0.29 0.58 0.87 1.16 1.45<br />
175 0.25 0.5 0.75 1.0 1.25<br />
200 0.22 0.43 0.65 0.87 1.08<br />
225 0.19 0.39 0.58 0.78 0.97<br />
250 0.17 0.35 0.52 0.7 0.87<br />
Women<br />
100 0.5 1.01 1.52 2.03 2.53<br />
125 0.4 0.8 1.2 1.62 2.02<br />
150 0.34 0.68 1.01 1.35 1.69<br />
175 0.29 0.58 0.87 1.17 1.46<br />
200 0.26 0.5 0.76 1.01 1.26<br />
225 0.22 0.45 0.68 0.91 1.13<br />
250 0.2 0.41 0.61 0.82 1.01<br />
Source: derived from Fox (1997).<br />
to one month. They found that the ethanol in beer was oxidised twice as fast as that in<br />
wine <strong>and</strong> seven times faster than that in whisky. Absorbed alcohol passes into the blood<br />
in 15–30 minutes if the stomach is empty, but in 1–3 hours if the stomach is full. There<br />
is a constant rate of metabolism of approximately 100 mg per hour per kg body weight.<br />
Thus an adult male weighing 70 kg drinking of 750 mL of beer on an empty stomach<br />
would not reach the French drinking limit of 0.8 g/L of blood.<br />
Alcohol should not be consumed with aspirin, which disables alcohol dehydrogenase<br />
(Roine et al. 1990).<br />
The predisposition to hangovers differs considerably between people with regard to<br />
the intensity of the condition in relation to how much alcohol has been taken. Furthermore<br />
it also depends on the type of drink. Thus, among the spirits, br<strong>and</strong>y has a more severe<br />
impact than whisky, with vodka having proportionately less tendency to cause hangovers.<br />
There has been much discussion but few rm conclusions concerning the prime causative<br />
agents of hangovers (Pradalier & Ollat 1991). They are believed partly to be due<br />
to a build-up of acetaldehyde produced via the oxidation of alcohol (Wall et al. 2000).<br />
The aldehyde interacts with components of brain cells to exert its effect. Congeners<br />
such as traces of methanol are also oxidised by alcohol <strong>and</strong> the resultant formaldehyde<br />
is even more unpleasant in its effects than acetaldehyde.<br />
Other compounds that may contribute to hangovers include some of the biogenic<br />
amines that are found in relatively small quantities in beer (see Table 6.4). These include<br />
15–200 µg/L histamine, 0.7–35.5 mg/L tyramine, 0.5–07 mg/L cadaverine, 3.1–5.6 mg/L<br />
putrescine <strong>and</strong> < 0.1–0.8 mg/L β-phenylethylamine (Cerutti et al. 1989).