Vectors <strong>and</strong> Gene Therapy | Head: Maria da Conceição Pedroso de LimaObjectivesThe Group <strong>of</strong> Vectors <strong>and</strong> Gene Therapy isdevoted to the design <strong>and</strong> development <strong>of</strong> carriers,including viral <strong>and</strong> non‐viral vectors, <strong>for</strong> nucleicacid <strong>and</strong> drug delivery aiming at their applicationin gene therapy <strong>and</strong> gene silencing approaches.Regarding the development <strong>of</strong> non‐viral vectors,the specific aims include the generation <strong>of</strong> novellipid‐based nanosystems <strong>for</strong> efficient intracellulardelivery <strong>of</strong> drugs <strong>and</strong> nucleic acids (plasmid DNA,antisense oligonucleotides or siRNAs) <strong>and</strong>evaluation <strong>of</strong> their potential in therapeuticapproaches <strong>for</strong> two major diseases: cancer <strong>and</strong>neurodegenerative disorders. For this purpose, ourresearch has been focused on the selection <strong>of</strong>appropriate lipids, lig<strong>and</strong>s <strong>and</strong> cell‐penetratingpeptides, <strong>and</strong> on the development <strong>of</strong> technology togenerate nanosystems with adequate features <strong>for</strong> invivo use, allowing targeting to specific cells ortissues <strong>and</strong> enhanced intracellular nucleic aciddelivery. In parallel, mechanistic studies on theinteraction <strong>of</strong> the developed systems with targetcells, including cell internalization <strong>and</strong> intracellulartrafficking, have also been addressed aiming attheir optimization <strong>for</strong> specific therapeuticapplications. Evaluation <strong>of</strong> the therapeutic activitymediated by the developed systems has beenper<strong>for</strong>med in several in vitro <strong>and</strong> in vivo models<strong>for</strong> cancer <strong>and</strong> neurodegenerative disorders, <strong>and</strong>also constitutes an important goal <strong>of</strong> this Group.Regarding viral vectors, the group aims atdeveloping <strong>and</strong> using viral vectors as atechnological plat<strong>for</strong>m to generate genetic models<strong>of</strong> neurodegenerative diseases, such as Machado‐Joseph disease, <strong>and</strong> to develop new moleculartherapeutic strategies involving gene transfer orsilencing <strong>of</strong> mutant genes by expression <strong>of</strong> shorthairpin RNAs.Main AchievementsRegarding the development <strong>of</strong> improved non‐viralgene delivery vectors, we demonstrated thecapacity <strong>of</strong> the S413‐PV cell‐penetrating peptide,either per se or in association with cationicliposomes, to very efficiently mediate theintracellular delivery <strong>of</strong> plasmid DNA.Regarding the evaluation <strong>of</strong> the developednanosystems in anticancer strategies, we haveshown that combination <strong>of</strong> antineoplastic agentswith suicide gene therapy mediated by albuminassociatedlipoplexes results in a remarkablesynergistic antitumor effect, highlighting thepotential <strong>of</strong> this approach <strong>for</strong> future applications inantitumoral therapy.We have also developed a novel lipid‐basednanosystem that has the potential to target themicroenvironment <strong>of</strong> human breast tumors at twodifferent levels: tumor cells <strong>and</strong> endothelial cells <strong>of</strong>tumor blood vessels. Such features led to adramatic improvement <strong>of</strong> cytotoxicity <strong>of</strong>encapsulated small molecular weight drug, ascompared to the non‐targeted <strong>for</strong>mulation. Thetargeting ability <strong>of</strong> the developed nanosystem wasconfirmed in tumor cells harvested from tumors <strong>of</strong>patients submitted to mastectomy.Concerning the potential <strong>of</strong> the developednanosystems in gene silencing approaches <strong>for</strong>neurodegenerative disorders, we achievedsignificant downregulation <strong>of</strong> gene expressionupon neuronal siRNA delivery mediated bytransferrin‐associated lipoplexes, both in vitro <strong>and</strong>in vivo. Moreover, promising results were obtainedregarding the application <strong>of</strong> these systems tomediate downregulation <strong>of</strong> specific pro‐apoptotictargets, which may prove useful in therapeuticapproaches to neuronal protection <strong>and</strong> repair.Using lentiviral vectors (LV) to transduce the ratbrain we overexpressed polyglutamine‐exp<strong>and</strong>edataxin‐3 in this way replicating Machado‐Josephdisease neuropathology. LVs also allowed the firstdemonstration <strong>of</strong> in vivo allele‐specific genesilencing. More recently we did not observetoxicity upon endogenous wild‐type ataxin‐3silencing <strong>and</strong> showed that LV‐mediated non‐allelespecificsilencing <strong>of</strong> ataxin‐3 is effective <strong>and</strong> welltolerated in vivo.38
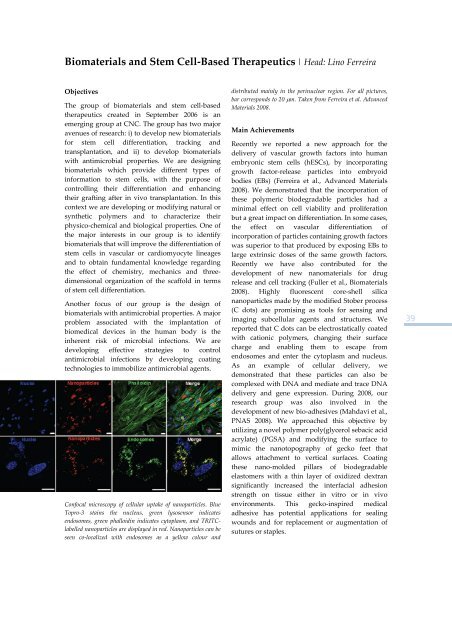
Biomaterials <strong>and</strong> Stem Cell‐Based Therapeutics | Head: Lino FerreiraObjectivesThe group <strong>of</strong> biomaterials <strong>and</strong> stem cell‐basedtherapeutics created in September 2006 is anemerging group at <strong>CNC</strong>. The group has two majoravenues <strong>of</strong> research: i) to develop new biomaterials<strong>for</strong> stem cell differentiation, tracking <strong>and</strong>transplantation, <strong>and</strong> ii) to develop biomaterialswith antimicrobial properties. We are designingbiomaterials which provide different types <strong>of</strong>in<strong>for</strong>mation to stem cells, with the purpose <strong>of</strong>controlling their differentiation <strong>and</strong> enhancingtheir grafting after in vivo transplantation. In thiscontext we are developing or modifying natural orsynthetic polymers <strong>and</strong> to characterize theirphysico‐chemical <strong>and</strong> biological properties. One <strong>of</strong>the major interests in our group is to identifybiomaterials that will improve the differentiation <strong>of</strong>stem cells in vascular or cardiomyocyte lineages<strong>and</strong> to obtain fundamental knowledge regardingthe effect <strong>of</strong> chemistry, mechanics <strong>and</strong> threedimensionalorganization <strong>of</strong> the scaffold in terms<strong>of</strong> stem cell differentiation.Another focus <strong>of</strong> our group is the design <strong>of</strong>biomaterials with antimicrobial properties. A majorproblem associated with the implantation <strong>of</strong>biomedical devices in the human body is theinherent risk <strong>of</strong> microbial infections. We aredeveloping effective strategies to controlantimicrobial infections by developing coatingtechnologies to immobilize antimicrobial agents.Confocal microscopy <strong>of</strong> cellular uptake <strong>of</strong> nanoparticles. BlueTopro‐3 stains the nucleus, green lysosensor indicatesendosomes, green phalloidin indicates cytoplasm, <strong>and</strong> TRITClabellednanoparticles are displayed in red. Nanoparticles can beseen co‐localized with endosomes as a yellow colour <strong>and</strong>distributed mainly in the perinuclear region. For all pictures,bar corresponds to 20 μm. Taken from Ferreira et al. AdvancedMaterials <strong>2008</strong>.Main AchievementsRecently we reported a new approach <strong>for</strong> thedelivery <strong>of</strong> vascular growth factors into humanembryonic stem cells (hESCs), by incorporatinggrowth factor‐release particles into embryoidbodies (EBs) (Ferreira et al., Advanced Materials<strong>2008</strong>). We demonstrated that the incorporation <strong>of</strong>these polymeric biodegradable particles had aminimal effect on cell viability <strong>and</strong> proliferationbut a great impact on differentiation. In some cases,the effect on vascular differentiation <strong>of</strong>incorporation <strong>of</strong> particles containing growth factorswas superior to that produced by exposing EBs tolarge extrinsic doses <strong>of</strong> the same growth factors.Recently we have also contributed <strong>for</strong> thedevelopment <strong>of</strong> new nanomaterials <strong>for</strong> drugrelease <strong>and</strong> cell tracking (Fuller et al., Biomaterials<strong>2008</strong>). Highly fluorescent core‐shell silicananoparticles made by the modified Stober process(C dots) are promising as tools <strong>for</strong> sensing <strong>and</strong>imaging subcellular agents <strong>and</strong> structures. Wereported that C dots can be electrostatically coatedwith cationic polymers, changing their surfacecharge <strong>and</strong> enabling them to escape fromendosomes <strong>and</strong> enter the cytoplasm <strong>and</strong> nucleus.As an example <strong>of</strong> cellular delivery, wedemonstrated that these particles can also becomplexed with DNA <strong>and</strong> mediate <strong>and</strong> trace DNAdelivery <strong>and</strong> gene expression. During <strong>2008</strong>, ourresearch group was also involved in thedevelopment <strong>of</strong> new bio‐adhesives (Mahdavi et al.,PNAS <strong>2008</strong>). We approached this objective byutilizing a novel polymer poly(glycerol sebacic acidacrylate) (PGSA) <strong>and</strong> modifying the surface tomimic the nanotopography <strong>of</strong> gecko feet thatallows attachment to vertical surfaces. Coatingthese nano‐molded pillars <strong>of</strong> biodegradableelastomers with a thin layer <strong>of</strong> oxidized dextransignificantly increased the interfacial adhesionstrength on tissue either in vitro or in vivoenvironments. This gecko‐inspired medicaladhesive has potential applications <strong>for</strong> sealingwounds <strong>and</strong> <strong>for</strong> replacement or augmentation <strong>of</strong>sutures or staples.39
- Page 7 and 8: General ObjectivesThe CNC major mis
- Page 11 and 12: OrganizationThe Center for Neurosci
- Page 13: Microbiology | Milton CostaMicrobio
- Page 16 and 17: per year) will be proposed by the g
- Page 18 and 19: Neuroprotection and Neurogenesis in
- Page 20 and 21: Retinal Dysfunction and Neurogenesi
- Page 22 and 23: Glutamatergic synapses | Head: Ana
- Page 24 and 25: Neuronal Cell Death and Neuroprotec
- Page 26 and 27: Molecular Mechanisms of Disease | H
- Page 28 and 29: PublicationsAgasse F, Bernardino L,
- Page 30 and 31: Santiago AR, Carvalho, CM, Carvalho
- Page 32 and 33: “Nano‐transportadores de base l
- Page 34 and 35: Vectors and Gene Therapy GroupM. Co
- Page 36 and 37: Molecular Systems Biology | Head: A
- Page 40 and 41: PublicationsAlves S, Nascimento‐F
- Page 43 and 44: Area C | Cell and Molecular Toxicol
- Page 45 and 46: Mitochondrial Toxicology and Pharma
- Page 47: Pharmacometrics GroupAmílcar Falc
- Page 50 and 51: Free Radicals and Antioxidants in B
- Page 52 and 53: Pharmacometrics | Head: Amílcar Fa
- Page 54 and 55: Correia S, Carvalho C, Santos MS, P
- Page 57 and 58: Area D | MicrobiologyCoordinator |
- Page 59: Microbiology of Extreme Environment
- Page 62 and 63: Medical Mycology - Yeast Research |
- Page 65 and 66: Area E | Biophysics and Biomedical
- Page 67 and 68: Inorganic Biochemistry and Molecula
- Page 69 and 70: Inorganic Biochemistry and Molecula
- Page 71 and 72: Cell Biophysics |Head: Luís Martin
- Page 73: Sobral AJFN, Justino LLG, Santos AC
- Page 76 and 77: Future PlansThere is an enormous we
- Page 78 and 79: Paula MotaSara M. Diniz Martins Lop
- Page 80 and 81: Biology of Reproduction and Human F
- Page 82 and 83: Insulin Resistance and Adipocyte |
- Page 85 and 86: Biomedical Inter‐Institutional Re
- Page 87 and 88: 3. Pediatric Research: metabolic di
- Page 89 and 90:
PublicationsSantos MJ, Cleto S, Men
- Page 91:
7. Research in brain cancer: geneti
- Page 94 and 95:
Grafting SVZ neural stem cell cultu
- Page 96 and 97:
Structure‐function analysis of th
- Page 98 and 99:
Anticancer Effects of of Phytochemi
- Page 100 and 101:
Investigaciones Biomédicas “Albe
- Page 102 and 103:
Participation in the organization o
- Page 104 and 105:
May 2008Member of the organizing co
- Page 106 and 107:
106
- Page 108 and 109:
Genome BiologyFebruary 25 ‐ 27Isa
- Page 110 and 111:
Seminars2008 Series | CNC Audithori
- Page 112 and 113:
13.6.2008Cells caught in the act: M
- Page 114 and 115:
5.12.2008Unexpected fate and functi
- Page 116 and 117:
Elisabete Ferreiro“Cross‐talk b
- Page 118 and 119:
Ana Isabel Vicente Rafael“Estudos
- Page 120 and 121:
Rosete Pais“Papilomavirus humano
- Page 122 and 123:
122
- Page 124 and 125:
124
- Page 126 and 127:
FLOW CYTOMETRY UNITHead of Unit: Is
- Page 128 and 129:
MASS SPECTROMETRY UNITHead of Unit:
- Page 130 and 131:
130
- Page 132 and 133:
Amino Acid AnalysisOur laboratory r
- Page 134 and 135:
2. Areas of Expertise / Research /
- Page 136 and 137:
Multiple SclerosisAn extension of t
- Page 138 and 139:
2.2. Centre for Bioavailability Stu
- Page 140 and 141:
StaffCoordinatorTice Macedo, MD, Ph
- Page 142 and 143:
142
- Page 144 and 145:
Compatible solutes from extremophil
- Page 146 and 147:
Proteases aspárticas secretadas em
- Page 148 and 149:
Mecanismos de plasticidade sinápti
- Page 150 and 151:
Clivagem dos transportadores vesicu
- Page 152 and 153:
ʺBIOINK ‐ Aprendizagem increment
- Page 154 and 155:
154
- Page 156 and 157:
Henrique Faneca (Auxiliar Inv., CNC
- Page 158 and 159:
Liliana Bernardino 100Luis Miguel E
- Page 160 and 161:
João Teixeira 100João Teodoro 100
- Page 162 and 163:
Patricia Henriques Domingues 100Pat
- Page 164 and 165:
ADMINISTRATIVE STAFFTime % at CNCAr
- Page 166 and 167:
Sandra Isabel M. Cardoso (Auxiliar
- Page 168 and 169:
Molecular Biotechnology and HealthE
- Page 170 and 171:
Cell and Molecular ToxicologyLeonor
- Page 172 and 173:
MicrobiologyMilton Costa, PhD, Coor
- Page 174 and 175:
Paulo Gameiro Guerreiro 100Pedro Co
- Page 176 and 177:
Marta Isabel Rodrigues Baptista 100
- Page 178:
Url: http://www.cnbc.pt | Email: in