Evaluating non-randomised intervention studies - NIHR Health ...
Evaluating non-randomised intervention studies - NIHR Health ...
Evaluating non-randomised intervention studies - NIHR Health ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
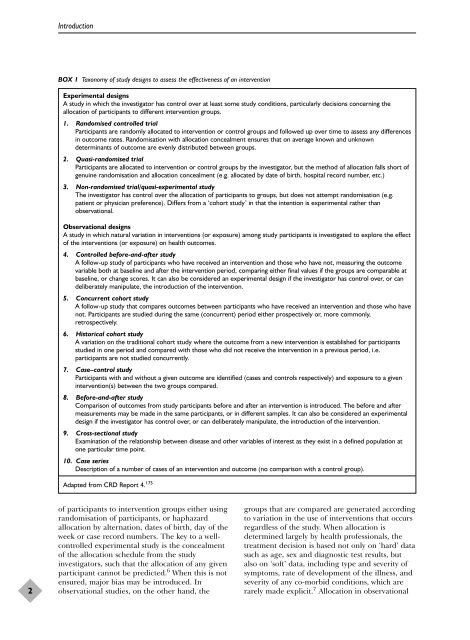
IntroductionBOX 1 Taxonomy of study designs to assess the effectiveness of an <strong>intervention</strong>Experimental designsA study in which the investigator has control over at least some study conditions, particularly decisions concerning theallocation of participants to different <strong>intervention</strong> groups.1. Randomised controlled trialParticipants are randomly allocated to <strong>intervention</strong> or control groups and followed up over time to assess any differencesin outcome rates. Randomisation with allocation concealment ensures that on average known and unknowndeterminants of outcome are evenly distributed between groups.2. Quasi-<strong>randomised</strong> trialParticipants are allocated to <strong>intervention</strong> or control groups by the investigator, but the method of allocation falls short ofgenuine randomisation and allocation concealment (e.g. allocated by date of birth, hospital record number, etc.)3. Non-<strong>randomised</strong> trial/quasi-experimental studyThe investigator has control over the allocation of participants to groups, but does not attempt randomisation (e.g.patient or physician preference). Differs from a ‘cohort study’ in that the intention is experimental rather thanobservational.Observational designsA study in which natural variation in <strong>intervention</strong>s (or exposure) among study participants is investigated to explore the effectof the <strong>intervention</strong>s (or exposure) on health outcomes.4. Controlled before-and-after studyA follow-up study of participants who have received an <strong>intervention</strong> and those who have not, measuring the outcomevariable both at baseline and after the <strong>intervention</strong> period, comparing either final values if the groups are comparable atbaseline, or change scores. It can also be considered an experimental design if the investigator has control over, or candeliberately manipulate, the introduction of the <strong>intervention</strong>.5. Concurrent cohort studyA follow-up study that compares outcomes between participants who have received an <strong>intervention</strong> and those who havenot. Participants are studied during the same (concurrent) period either prospectively or, more commonly,retrospectively.6. Historical cohort studyA variation on the traditional cohort study where the outcome from a new <strong>intervention</strong> is established for participantsstudied in one period and compared with those who did not receive the <strong>intervention</strong> in a previous period, i.e.participants are not studied concurrently.7. Case–control studyParticipants with and without a given outcome are identified (cases and controls respectively) and exposure to a given<strong>intervention</strong>(s) between the two groups compared.8. Before-and-after studyComparison of outcomes from study participants before and after an <strong>intervention</strong> is introduced. The before and aftermeasurements may be made in the same participants, or in different samples. It can also be considered an experimentaldesign if the investigator has control over, or can deliberately manipulate, the introduction of the <strong>intervention</strong>.9. Cross-sectional studyExamination of the relationship between disease and other variables of interest as they exist in a defined population atone particular time point.10. Case seriesDescription of a number of cases of an <strong>intervention</strong> and outcome (no comparison with a control group).Adapted from CRD Report 4. 1752of participants to <strong>intervention</strong> groups either usingrandomisation of participants, or haphazardallocation by alternation, dates of birth, day of theweek or case record numbers. The key to a wellcontrolledexperimental study is the concealmentof the allocation schedule from the studyinvestigators, such that the allocation of any givenparticipant cannot be predicted. 6 When this is notensured, major bias may be introduced. Inobservational <strong>studies</strong>, on the other hand, thegroups that are compared are generated accordingto variation in the use of <strong>intervention</strong>s that occursregardless of the study. When allocation isdetermined largely by health professionals, thetreatment decision is based not only on ‘hard’ datasuch as age, sex and diagnostic test results, butalso on ‘soft’ data, including type and severity ofsymptoms, rate of development of the illness, andseverity of any co-morbid conditions, which arerarely made explicit. 7 Allocation in observational