computer modeling in molecular biology.pdf
computer modeling in molecular biology.pdf
computer modeling in molecular biology.pdf
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
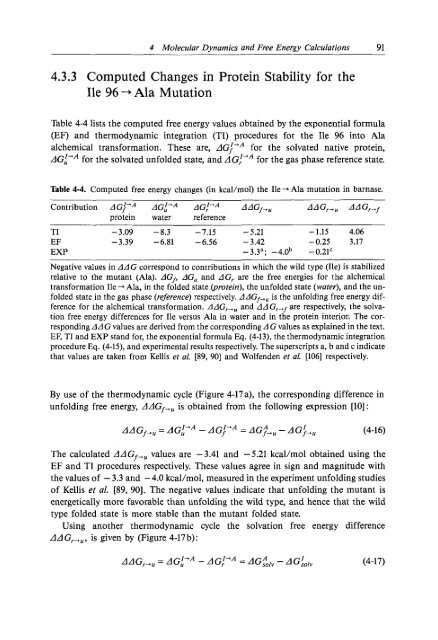
4 Molecular Dynamics and Free Energy Calculations 914.3.3 Computed Changes <strong>in</strong> Prote<strong>in</strong> Stability for theIle 96-+Ala MutationTable 4-4 lists the computed free energy values obta<strong>in</strong>ed by the exponential formula(EF) and thermodynamic <strong>in</strong>tegration (TI) procedures for the Ile 96 <strong>in</strong>to Alaalchemical transformation. These are, ~lGf”~ for the solvated native prote<strong>in</strong>,LIGL’~ for the solvated unfolded state, and d GI’A for the gas phase reference state.Table 4-4. Computed free energy changes (<strong>in</strong> kcal/aol) the Ile --* Ala mutation <strong>in</strong> barnase.Contribution AGf-A AGf’A AAGf,,, AAG,,, AAG,,fprote<strong>in</strong> water referenceTI -3.09 -8.3~~-7.15 - 5.21 -1.15 4.06EF -3.39 -6.81 -6.56 - 3.42 -0.25 3.17EXP -3.3a; -4.0b -0.21‘Negative values <strong>in</strong> AAG correspond to contributions <strong>in</strong> which the wild type (Ile) is stabilizedrelative to the mutant (Ala). AGf, AG, and AG, are the free energies for the alchemicaltransformation Ile + Ala, <strong>in</strong> the folded state (prote<strong>in</strong>), the unfolded state (water), and the unfoldedstate <strong>in</strong> the gas phase (reference) respectively. AAGf-u is the unfold<strong>in</strong>g free energy differencefor the alchemical transformation. AAG,,, and AA G,,f are respectively, the solvationfree energy differences for Ile versus Ala <strong>in</strong> water and <strong>in</strong> the prote<strong>in</strong> <strong>in</strong>terior. The correspond<strong>in</strong>gAAG values are derived from the correspond<strong>in</strong>g AG values as expla<strong>in</strong>ed <strong>in</strong> the text.EF, TI and EXP stand for, the exponential formula Eq. (4-13), the thermodynamic <strong>in</strong>tegrationprocedure Eq. (4-15), and experimental results respectively. The superscripts a, b and c <strong>in</strong>dicatethat values are taken from Kellis et al. [89, 901 and Wolfenden et 01. [lo61 respectively.By use of the thermodynamic cycle (Figure 4-17 a), the correspond<strong>in</strong>g difference <strong>in</strong>unfold<strong>in</strong>g free energy, d ~lG~,~ is obta<strong>in</strong>ed from the follow<strong>in</strong>g expression [lo] :AAG~,, = AG,”~ - LIG~”~= AG&, - AG;,,, (4-16)The calculated LIL~G~+~ values are -3.41 and -5.21 kcal/mol obta<strong>in</strong>ed us<strong>in</strong>g theEF and TI procedures respectively. These values agree <strong>in</strong> sign and magnitude withthe values of - 3.3 and - 4.0 kcal/mol, measured <strong>in</strong> the experiment unfold<strong>in</strong>g studiesof Kellis et al. [89, 901. The negative values <strong>in</strong>dicate that unfold<strong>in</strong>g the mutant isenergetically more favorable than unfold<strong>in</strong>g the wild type, and hence that the wildtype folded state is more stable than the mutant folded state.Us<strong>in</strong>g another thermodynamic cycle the solvation free energy differencedAG,,,, is given by (Figure 4-17b):AAG,,, = AG;’* - LIG~+ = AG$, - AG:~,, (4-17)