Role of the ubiquitin-like modifier FAT10 in protein degradation and ...
Role of the ubiquitin-like modifier FAT10 in protein degradation and ...
Role of the ubiquitin-like modifier FAT10 in protein degradation and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Introduction<br />
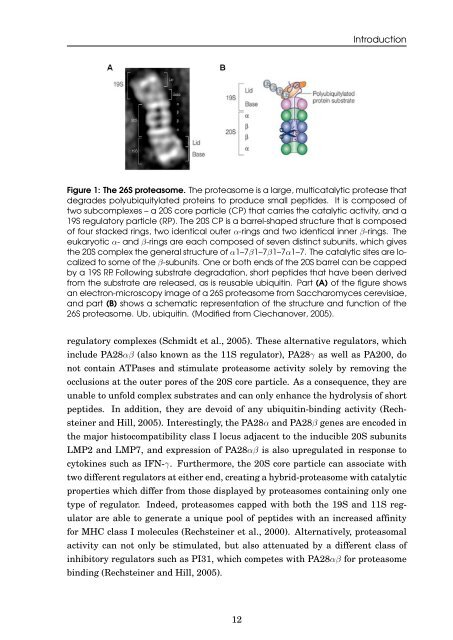
Figure 1: The 26S proteasome. The proteasome is a large, multicatalytic protease that<br />
degrades polyubiquitylated prote<strong>in</strong>s to produce small peptides. It is composed <strong>of</strong><br />
two subcomplexes – a 20S core particle (CP) that carries <strong>the</strong> catalytic activity, <strong>and</strong> a<br />
19S regulatory particle (RP). The 20S CP is a barrel-shaped structure that is composed<br />
<strong>of</strong> four stacked r<strong>in</strong>gs, two identical outer α-r<strong>in</strong>gs <strong>and</strong> two identical <strong>in</strong>ner β-r<strong>in</strong>gs. The<br />
eukaryotic α- <strong>and</strong> β-r<strong>in</strong>gs are each composed <strong>of</strong> seven dist<strong>in</strong>ct subunits, which gives<br />
<strong>the</strong> 20S complex <strong>the</strong> general structure <strong>of</strong> α1–7β1–7β1–7α1–7. The catalytic sites are localized<br />
to some <strong>of</strong> <strong>the</strong> β-subunits. One or both ends <strong>of</strong> <strong>the</strong> 20S barrel can be capped<br />
by a 19S RP. Follow<strong>in</strong>g substrate <strong>degradation</strong>, short peptides that have been derived<br />
from <strong>the</strong> substrate are released, as is reusable <strong>ubiquit<strong>in</strong></strong>. Part (A) <strong>of</strong> <strong>the</strong> figure shows<br />
an electron-microscopy image <strong>of</strong> a 26S proteasome from Saccharomyces cerevisiae,<br />
<strong>and</strong> part (B) shows a schematic representation <strong>of</strong> <strong>the</strong> structure <strong>and</strong> function <strong>of</strong> <strong>the</strong><br />
26S proteasome. Ub, <strong>ubiquit<strong>in</strong></strong>. (Modified from Ciechanover, 2005).<br />
regulatory complexes (Schmidt et al., 2005). These alternative regulators, which<br />
<strong>in</strong>clude PA28αβ (also known as <strong>the</strong> 11S regulator), PA28γ as well as PA200, do<br />
not conta<strong>in</strong> ATPases <strong>and</strong> stimulate proteasome activity solely by remov<strong>in</strong>g <strong>the</strong><br />
occlusions at <strong>the</strong> outer pores <strong>of</strong> <strong>the</strong> 20S core particle. As a consequence, <strong>the</strong>y are<br />
unable to unfold complex substrates <strong>and</strong> can only enhance <strong>the</strong> hydrolysis <strong>of</strong> short<br />
peptides. In addition, <strong>the</strong>y are devoid <strong>of</strong> any <strong>ubiquit<strong>in</strong></strong>-b<strong>in</strong>d<strong>in</strong>g activity (Rech-<br />
ste<strong>in</strong>er <strong>and</strong> Hill, 2005). Interest<strong>in</strong>gly, <strong>the</strong> PA28α <strong>and</strong> PA28β genes are encoded <strong>in</strong><br />
<strong>the</strong> major histocompatibility class I locus adjacent to <strong>the</strong> <strong>in</strong>ducible 20S subunits<br />
LMP2 <strong>and</strong> LMP7, <strong>and</strong> expression <strong>of</strong> PA28αβ is also upregulated <strong>in</strong> response to<br />
cytok<strong>in</strong>es such as IFN-γ. Fur<strong>the</strong>rmore, <strong>the</strong> 20S core particle can associate with<br />
two different regulators at ei<strong>the</strong>r end, creat<strong>in</strong>g a hybrid-proteasome with catalytic<br />
properties which differ from those displayed by proteasomes conta<strong>in</strong><strong>in</strong>g only one<br />
type <strong>of</strong> regulator. Indeed, proteasomes capped with both <strong>the</strong> 19S <strong>and</strong> 11S reg-<br />
ulator are able to generate a unique pool <strong>of</strong> peptides with an <strong>in</strong>creased aff<strong>in</strong>ity<br />
for MHC class I molecules (Rechste<strong>in</strong>er et al., 2000). Alternatively, proteasomal<br />
activity can not only be stimulated, but also attenuated by a different class <strong>of</strong><br />
<strong>in</strong>hibitory regulators such as PI31, which competes with PA28αβ for proteasome<br />
b<strong>in</strong>d<strong>in</strong>g (Rechste<strong>in</strong>er <strong>and</strong> Hill, 2005).<br />
12