Role of the ubiquitin-like modifier FAT10 in protein degradation and ...
Role of the ubiquitin-like modifier FAT10 in protein degradation and ...
Role of the ubiquitin-like modifier FAT10 in protein degradation and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 3<br />
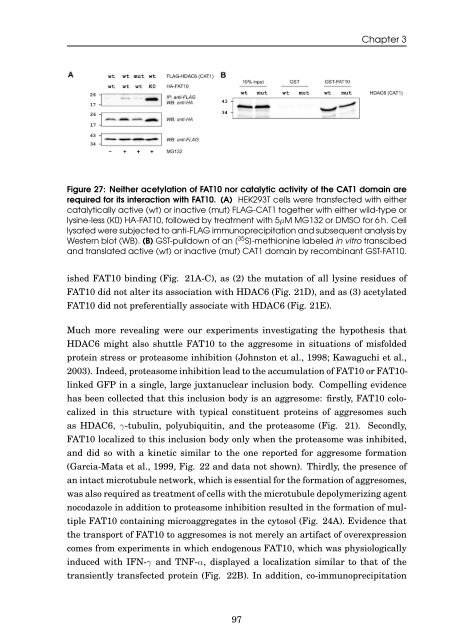
Figure 27: Nei<strong>the</strong>r acetylation <strong>of</strong> <strong>FAT10</strong> nor catalytic activity <strong>of</strong> <strong>the</strong> CAT1 doma<strong>in</strong> are<br />
required for its <strong>in</strong>teraction with <strong>FAT10</strong>. (A) HEK293T cells were transfected with ei<strong>the</strong>r<br />
catalytically active (wt) or <strong>in</strong>active (mut) FLAG-CAT1 toge<strong>the</strong>r with ei<strong>the</strong>r wild-type or<br />
lys<strong>in</strong>e-less (K0) HA-<strong>FAT10</strong>, followed by treatment with 5µM MG132 or DMSO for 6 h. Cell<br />
lysated were subjected to anti-FLAG immunoprecipitation <strong>and</strong> subsequent analysis by<br />
Western blot (WB). (B) GST-pulldown <strong>of</strong> an [ 35 S]-methion<strong>in</strong>e labeled <strong>in</strong> vitro transcibed<br />
<strong>and</strong> translated active (wt) or <strong>in</strong>active (mut) CAT1 doma<strong>in</strong> by recomb<strong>in</strong>ant GST-<strong>FAT10</strong>.<br />
ished <strong>FAT10</strong> b<strong>in</strong>d<strong>in</strong>g (Fig. 21A-C), as (2) <strong>the</strong> mutation <strong>of</strong> all lys<strong>in</strong>e residues <strong>of</strong><br />
<strong>FAT10</strong> did not alter its association with HDAC6 (Fig. 21D), <strong>and</strong> as (3) acetylated<br />
<strong>FAT10</strong> did not preferentially associate with HDAC6 (Fig. 21E).<br />
Much more reveal<strong>in</strong>g were our experiments <strong>in</strong>vestigat<strong>in</strong>g <strong>the</strong> hypo<strong>the</strong>sis that<br />
HDAC6 might also shuttle <strong>FAT10</strong> to <strong>the</strong> aggresome <strong>in</strong> situations <strong>of</strong> misfolded<br />
prote<strong>in</strong> stress or proteasome <strong>in</strong>hibition (Johnston et al., 1998; Kawaguchi et al.,<br />
2003). Indeed, proteasome <strong>in</strong>hibition lead to <strong>the</strong> accumulation <strong>of</strong> <strong>FAT10</strong> or <strong>FAT10</strong>-<br />
l<strong>in</strong>ked GFP <strong>in</strong> a s<strong>in</strong>gle, large juxtanuclear <strong>in</strong>clusion body. Compell<strong>in</strong>g evidence<br />
has been collected that this <strong>in</strong>clusion body is an aggresome: firstly, <strong>FAT10</strong> colo-<br />
calized <strong>in</strong> this structure with typical constituent prote<strong>in</strong>s <strong>of</strong> aggresomes such<br />
as HDAC6, γ-tubul<strong>in</strong>, poly<strong>ubiquit<strong>in</strong></strong>, <strong>and</strong> <strong>the</strong> proteasome (Fig. 21). Secondly,<br />
<strong>FAT10</strong> localized to this <strong>in</strong>clusion body only when <strong>the</strong> proteasome was <strong>in</strong>hibited,<br />
<strong>and</strong> did so with a k<strong>in</strong>etic similar to <strong>the</strong> one reported for aggresome formation<br />
(Garcia-Mata et al., 1999, Fig. 22 <strong>and</strong> data not shown). Thirdly, <strong>the</strong> presence <strong>of</strong><br />
an <strong>in</strong>tact microtubule network, which is essential for <strong>the</strong> formation <strong>of</strong> aggresomes,<br />
was also required as treatment <strong>of</strong> cells with <strong>the</strong> microtubule depolymeriz<strong>in</strong>g agent<br />
nocodazole <strong>in</strong> addition to proteasome <strong>in</strong>hibition resulted <strong>in</strong> <strong>the</strong> formation <strong>of</strong> mul-<br />
tiple <strong>FAT10</strong> conta<strong>in</strong><strong>in</strong>g microaggregates <strong>in</strong> <strong>the</strong> cytosol (Fig. 24A). Evidence that<br />
<strong>the</strong> transport <strong>of</strong> <strong>FAT10</strong> to aggresomes is not merely an artifact <strong>of</strong> overexpression<br />
comes from experiments <strong>in</strong> which endogenous <strong>FAT10</strong>, which was physiologically<br />
<strong>in</strong>duced with IFN-γ <strong>and</strong> TNF-α, displayed a localization similar to that <strong>of</strong> <strong>the</strong><br />
transiently transfected prote<strong>in</strong> (Fig. 22B). In addition, co-immunoprecipitation<br />
97