- Page 3 and 4:
Hak Cipta pada Departemen Pendidika
- Page 5 and 6:
Petunjuk Penggunaan Buku Buku Prakt
- Page 7 and 8:
Daftar Isi Kata Sambutan • iii Pe
- Page 9 and 10:
viii
- Page 11 and 12:
2 Soal Pramateri 1. Berapakah volum
- Page 13 and 14:
4 Kupas Tuntas Fraksi mol suatu lar
- Page 15 and 16:
6 Legenda Kimia Marie Francois Raou
- Page 17 and 18:
Tekanan 8 Kupas Tuntas Diagram P-T
- Page 19 and 20:
Kata Kunci Penurunan titik beku 10
- Page 21 and 22:
Kata Kunci • Membran semipermeabe
- Page 23 and 24:
14 F a k t a K i m i a Desalinasi A
- Page 25 and 26:
Kata Kunci • Faktor Van’t Hoff
- Page 27 and 28:
18 Kupas Tuntas Agar 10 kg air tida
- Page 29 and 30:
20 Sifat koligatif larutan Kaji Dir
- Page 31 and 32:
17. Tekanan osmotik suatu larutan y
- Page 33 and 34:
24 Soal Pramateri 1. Bagaimanakah k
- Page 35 and 36:
26 Kupas Tuntas Reaksi redoks berik
- Page 37 and 38:
Kata Kunci • Jumlah elektron •
- Page 39 and 40:
30 Legenda Kimia Alessandro Volta (
- Page 41 and 42:
32 Anda Harus Ingat Fungsi jembatan
- Page 43 and 44:
34 Gambar 2.3 Pengukuran harga pote
- Page 45 and 46:
36 Kupas Tuntas Diketahui: Zn(s) +
- Page 47 and 48:
38 Output H 2 (g) O 2 (g) Anode Kat
- Page 49 and 50:
40 Kupas Tuntas Larutan CaCl 2 deng
- Page 51 and 52:
42 Ag Ag + Ag + AgNO 3 (aq) e Sendo
- Page 53 and 54:
Kata Kunci • Korosi • Pencegaha
- Page 55 and 56:
Evaluasi Materi Bab 2 A. Pilihlah s
- Page 57 and 58:
A. 44,8 L D. 5,6 L B. 22,4 L E. 2,8
- Page 59 and 60:
50 Soal Pramateri 1. Apakah perbeda
- Page 61 and 62:
52 Sumber: Chemisry for You, 2001 G
- Page 63 and 64:
Kata Kunci • Sifat fisis • Sifa
- Page 65 and 66:
56 Sumber: Chemistry (Ann and Patri
- Page 67 and 68:
58 Gambar 3.10 Warna nyala unsur-un
- Page 69 and 70:
60 Sumber: Chemistry (Chang), 2002
- Page 71 and 72:
62 Soal Penguasaan Materi 3.2 Kerja
- Page 73 and 74:
64 Tantangan Kimia Sumber: Chemistr
- Page 75 and 76:
66 Sumber: Science Discovery, 1991
- Page 77 and 78:
68 Legenda Kimia Sir Henry Bessemer
- Page 79 and 80:
70 Praktis Belajar Kimia untuk Kela
- Page 81 and 82:
72 Rangkuman 1. Unsur-unsur kimia d
- Page 83 and 84:
Evaluasi Materi Bab 3 A. Pilihlah s
- Page 85 and 86:
7. Suatu bijih besi mengandung 80%
- Page 87 and 88:
78 Praktis Belajar Kimia untuk Kela
- Page 89 and 90:
80 Soal Pramateri 1. Apakah yang An
- Page 91 and 92:
82 Legenda Kimia Antoine Becquerel
- Page 93 and 94:
84 Gambar 4.2 Deret peluruhan radio
- Page 95 and 96:
• Fisi • Fusi 86 Legenda Kimia
- Page 97 and 98:
88 Tantangan Kimia Dapatkah Anda se
- Page 99 and 100:
90 F a k t a K i m i a Reaksi Beran
- Page 101 and 102:
1. Isotop adalah unsur-unsur dengan
- Page 103 and 104:
Evaluasi Materi Bab 4 A. Pilihlah s
- Page 105 and 106:
1. Suatu larutan gliserin C 3 H 5 (
- Page 107 and 108:
25. Pada reaksi transmutasi, 14 7 9
- Page 109 and 110:
1. Apakah yang dimaksud dengan seny
- Page 111 and 112:
102 Gambar 5.4 Senyawa aldehid memi
- Page 113 and 114:
104 Anda Harus Ingat Senyawa alkoho
- Page 115 and 116:
Kata Kunci • Alkil • Rantai cab
- Page 117 and 118:
108 Kupas Tuntas Senyawa yang terma
- Page 119 and 120:
110 Anda Harus Ingat Senyawa eter m
- Page 121 and 122:
112 Anda Harus Ingat Senyawa aldehi
- Page 123 and 124:
114 Anda Harus Ingat Senyawa asam k
- Page 125 and 126:
116 Anda Harus Ingat Senyawa ester
- Page 127 and 128:
118 b. Praktis Belajar Kimia untuk
- Page 129 and 130:
120 Anda Harus Ingat Haloalkana ada
- Page 131 and 132:
122 Anda Harus Ingat Senyawa-senyaw
- Page 133:
124 Anda Harus Ingat Isomer gugus f
- Page 136 and 137:
Langkah Kerja 1. Masukkanlah 2 mL f
- Page 138 and 139:
Soal Penguasaan Materi 5.4 Kerjakan
- Page 140:
Senyawa karbon turunan alkana Kaji
- Page 145 and 146: 136 Soal Pramateri 1. Apakah keunik
- Page 147 and 148: Kata Kunci • Benzena • Substitu
- Page 149 and 150: 140 Anda Harus Ingat Terdapat tiga
- Page 151 and 152: 142 Kupas Tuntas Turunan benzena ya
- Page 153 and 154: 144 Gambar 6.4 Semir sepatu mengand
- Page 155 and 156: Kerjakanlah di dalam buku latihan A
- Page 157 and 158: Evaluasi Materi Bab 6 A. Pilihlah s
- Page 159 and 160: Soal Tantangan 1. 2-fenil etanol da
- Page 161 and 162: 152 Soal Pramateri 1. Apakah yang A
- Page 163 and 164: Kata Kunci • Polimer • Polimeri
- Page 165 and 166: 156 Kupas Tuntas Suatu senyawa dapa
- Page 167 and 168: Kata Kunci • Ikatan glikosida •
- Page 169 and 170: 160 β 2 α 2 Gambar 7.3 Struktur m
- Page 171 and 172: Kerjakanlah di dalam buku latihan A
- Page 173 and 174: 164 Sumber: www.depkes.go.id Gambar
- Page 175 and 176: Gambar 7.7 Margarin merupakan conto
- Page 177 and 178: 168 Makromolekul Lemak dan minyak L
- Page 179 and 180: B. Jawablah pertanyaan berikut deng
- Page 181 and 182: 172 Praktis Belajar Kimia untuk Kel
- Page 183 and 184: 14. Rumus molekul berikut yang tida
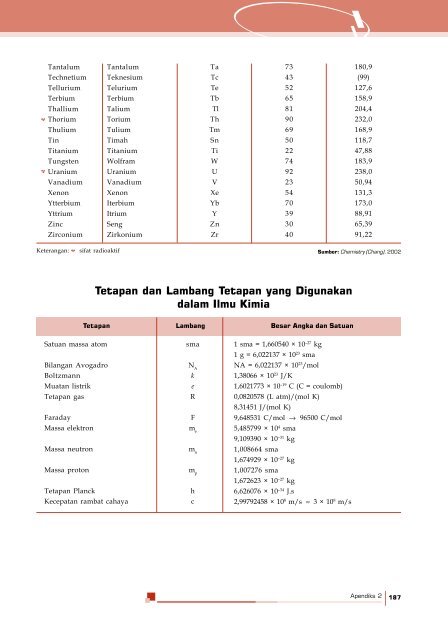
- Page 185 and 186: 14. Pernyataan berikut yang tepat u
- Page 187 and 188: Bab 1 Soal Penguasaan Materi 1.1 1.
- Page 189 and 190: Evaluasi Materi Bab 4 A. Pilihan ga
- Page 191 and 192: 182 d. e. Cl—C—CH O 3 H f. CH3
- Page 193 and 194: 2. a. asam benzoat b. nitrobenzena
- Page 195: Praktis Belajar Kimia untuk Kelas X
- Page 199 and 200: H haloalkana: suatu senyawa alkana
- Page 201 and 202: A 192 Indeks adisi 133, 154, 155, 1
- Page 203: 194 Daftar Pustaka Achmad Hiskia, T