download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
76 CHAPTER 2. ATMOSPHERE AND REMOTE SENSING<br />
2.5.1 Modeling iodide – iodate speciation in atmospheric aerosol<br />
Susanne Pechtl, Guy Schmitz (Faculté des Sciences Appliquées, Universitée Libre de Bruxelles,<br />
Belgium), Roland von Glasow<br />
Abstract The speciation of iodine in atmospheric aerosol is currently poorly understood. Models<br />
predict negligible iodide concentrations, but accumulation of iodate in aerosol, both of which is not<br />
confirmed by recent measurements. An updated aqueous phase iodine chemistry scheme for use in<br />
atmospheric models was developed, which improves the agreement with measurements significantly.<br />
HIO 3<br />
IO<br />
ICl 2 -<br />
IClBr -<br />
ICl<br />
HOCl<br />
HOBr<br />
HOI<br />
Cl -<br />
H + Br -<br />
H +<br />
IBr 2 -<br />
HI<br />
HIO 2<br />
IBr<br />
I -<br />
H +<br />
H2O2 HOCl HOBr<br />
H +<br />
HIO2 H +<br />
HSO 3 -<br />
DOM?<br />
HOCl<br />
SO 3 -<br />
I 2<br />
HOBr<br />
H +<br />
O 3<br />
H<br />
HOI<br />
+ IBr<br />
ICl<br />
H +<br />
IO 3 -<br />
HSO 3 -<br />
I -<br />
aerosol<br />
gas phase<br />
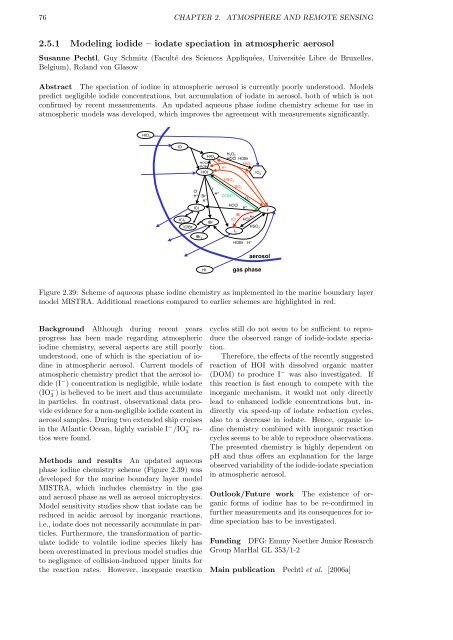
Figure 2.39: Scheme of aqueous phase iodine chemistry as implemented in the marine boundary layer<br />
model MISTRA. Additional reactions compared to earlier schemes are highlighted in red.<br />
Background Although during recent years<br />
progress has been made regarding atmospheric<br />
iodine chemistry, several aspects are still poorly<br />
understood, one of which is the speciation of iodine<br />
in atmospheric aerosol. Current models of<br />
atmospheric chemistry predict that the aerosol iodide<br />
(I− ) concentration is negligible, while iodate<br />
(IO − 3<br />
) is believed to be inert and thus accumulate<br />
in particles. In contrast, observational data provide<br />
evidence for a non-negligible iodide content in<br />
aerosol samples. During two extended ship cruises<br />
in the Atlantic Ocean, highly variable I − /IO − 3 ratios<br />
were found.<br />
Methods and results An updated aqueous<br />
phase iodine chemistry scheme (Figure 2.39) was<br />
developed for the marine boundary layer model<br />
MISTRA, which includes chemistry in the gas<br />
and aerosol phase as well as aerosol microphysics.<br />
Model sensitivity studies show that iodate can be<br />
reduced in acidic aerosol by inorganic reactions,<br />
i.e., iodate does not necessarily accumulate in particles.<br />
Furthermore, the transformation of particulate<br />
iodide to volatile iodine species likely has<br />
been overestimated in previous model studies due<br />
to negligence of collision-induced upper limits for<br />
the reaction rates. However, inorganic reaction<br />
cycles still do not seem to be sufficient to reproduce<br />
the observed range of iodide-iodate speciation.<br />
Therefore, the effects of the recently suggested<br />
reaction of HOI with dissolved organic matter<br />
(DOM) to produce I − was also investigated. If<br />
this reaction is fast enough to compete with the<br />
inorganic mechanism, it would not only directly<br />
lead to enhanced iodide concentrations but, indirectly<br />
via speed-up of iodate reduction cycles,<br />
also to a decrease in iodate. Hence, organic iodine<br />
chemistry combined with inorganic reaction<br />
cycles seems to be able to reproduce observations.<br />
The presented chemistry is highly dependent on<br />
pH and thus offers an explanation for the large<br />
observed variability of the iodide-iodate speciation<br />
in atmospheric aerosol.<br />
Outlook/Future work The existence of organic<br />
forms of iodine has to be re-confirmed in<br />
further measurements and its consequences for iodine<br />
speciation has to be investigated.<br />
Funding DFG: Emmy Noether Junior Research<br />
Group MarHal GL 353/1-2<br />
Main publication Pechtl et al. [2006a]