download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2.5. MARHAL - MODELING OF MARINE AND HALOGEN CHEMISTRY 79<br />
2.5.4 Importance of the surface reaction OH + Cl − on sea salt aerosol for<br />
the chemistry of the marine boundary layer - a model study<br />
Roland von Glasow<br />
Abstract The implications for the chemistry of the marine boundary layer (MBL) of the reaction<br />
of OH with Cl − on the surface of sea salt aerosol producing gas phase Cl2 and particulate OH − have<br />
been investigated with a numerical model. They were found to be very minor in contradiction to<br />
previous suggestions in the literature.<br />
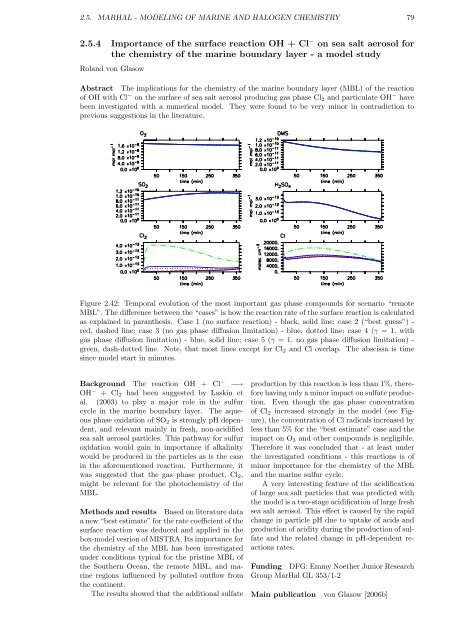
Figure 2.42: Temporal evolution of the most important gas phase compounds for scenario “remote<br />
MBL”. The difference between the “cases” is how the reaction rate of the surface reaction is calculated<br />
as explained in paranthesis. Case 1 (no surface reaction) - black, solid line; case 2 (“best guess”) -<br />
red, dashed line; case 3 (no gas phase diffusion limitation) - blue, dotted line; case 4 (γ = 1, with<br />
gas phase diffusion limitation) - blue, solid line; case 5 (γ = 1, no gas phase diffusion limitation) -<br />
green, dash-dotted line. Note, that most lines except for Cl2 and Cl overlap. The abscissa is time<br />
since model start in minutes.<br />
Background The reaction OH + Cl − −→<br />
OH − + Cl2 had been suggested by Laskin et<br />
al. (2003) to play a major role in the sulfur<br />
cycle in the marine boundary layer. The aqueous<br />
phase oxidation of SO2 is strongly pH dependent,<br />
and relevant mainly in fresh, non-acidified<br />
sea salt aerosol particles. This pathway for sulfur<br />
oxidation would gain in importance if alkalinity<br />
would be produced in the particles as is the case<br />
in the aforementioned reaction. Furthermore, it<br />
was suggested that the gas phase product, Cl2,<br />
might be relevant for the photochemistry of the<br />
MBL.<br />
Methods and results Based on literature data<br />
a new “best estimate” for the rate coefficient of the<br />
surface reaction was deduced and applied in the<br />
box-model vesrion of MISTRA. Its importance for<br />
the chemistry of the MBL has been investigated<br />
under conditions typical for the pristine MBL of<br />
the Southern Ocean, the remote MBL, and marine<br />
regions influenced by polluted outflow from<br />
the continent.<br />
The results showed that the additional sulfate<br />
production by this reaction is less than 1%, therefore<br />
having only a minor impact on sulfate production.<br />
Even though the gas phase concentration<br />
of Cl2 increased strongly in the model (see Figure),<br />
the concentration of Cl radicals increased by<br />
less than 5% for the “best estimate” case and the<br />
impact on O3 and other compounds is negligible.<br />
Therefore it was concluded that - at least under<br />
the investigated conditions - this reactions is of<br />
minor importance for the chemistry of the MBL<br />
and the marine sulfur cycle.<br />
A very interesting feature of the acidification<br />
of large sea salt particles that was predicted with<br />
the model is a two-stage acidification of large fresh<br />
sea salt aerosol. This effect is caused by the rapid<br />
change in particle pH due to uptake of acids and<br />
production of acidity during the production of sulfate<br />
and the related change in pH-dependent reactions<br />
rates.<br />
Funding DFG: Emmy Noether Junior Research<br />
Group MarHal GL 353/1-2<br />
Main publication von Glasow [2006b]