download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
78 CHAPTER 2. ATMOSPHERE AND REMOTE SENSING<br />
2.5.3 Modeling organic surface films on Atmospheric Aerosol Particles and<br />
their Influence on Chemistry<br />
Linda Smoydzin, Roland von Glasow<br />
Abstract Organic material from the ocean’s surface can be incorporated into sea salt aerosol particles<br />
often producing a surface film on the aerosol. Such an organic coating can reduce the mass<br />
transfer between the gas phase and the aerosol phase influencing sea salt chemistry in the marine<br />
atmosphere.<br />
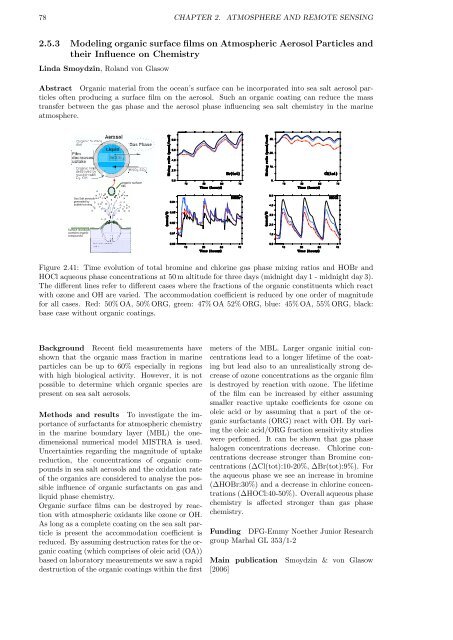
Figure 2.41: Time evolution of total bromine and chlorine gas phase mixing ratios and HOBr and<br />
HOCl aqueous phase concentrations at 50 m altitude for three days (midnight day 1 - midnight day 3).<br />
The different lines refer to different cases where the fractions of the organic constituents which react<br />
with ozone and OH are varied. The accommodation coefficient is reduced by one order of magnitude<br />
for all cases. Red: 50% OA, 50% ORG, green: 47% OA 52% ORG, blue: 45% OA, 55% ORG, black:<br />
base case without organic coatings.<br />
Background Recent field measurements have<br />
shown that the organic mass fraction in marine<br />
particles can be up to 60% especially in regions<br />
with high biological activity. However, it is not<br />
possible to determine which organic species are<br />
present on sea salt aerosols.<br />
Methods and results To investigate the importance<br />
of surfactants for atmospheric chemistry<br />
in the marine boundary layer (MBL) the onedimensional<br />
numerical model MISTRA is used.<br />
Uncertainties regarding the magnitude of uptake<br />
reduction, the concentrations of organic compounds<br />
in sea salt aerosols and the oxidation rate<br />
of the organics are considered to analyse the possible<br />
influence of organic surfactants on gas and<br />
liquid phase chemistry.<br />
Organic surface films can be destroyed by reaction<br />
with atmospheric oxidants like ozone or OH.<br />
As long as a complete coating on the sea salt particle<br />
is present the accommodation coefficient is<br />
reduced. By assuming destruction rates for the organic<br />
coating (which comprises of oleic acid (OA))<br />
based on laboratory measurements we saw a rapid<br />
destruction of the organic coatings within the first<br />
meters of the MBL. Larger organic initial concentrations<br />
lead to a longer lifetime of the coating<br />
but lead also to an unrealistically strong decrease<br />
of ozone concentrations as the organic film<br />
is destroyed by reaction with ozone. The lifetime<br />
of the film can be increased by either assuming<br />
smaller reactive uptake coefficients for ozone on<br />
oleic acid or by assuming that a part of the organic<br />
surfactants (ORG) react with OH. By variing<br />
the oleic acid/ORG fraction sensitivity studies<br />
were perfomed. It can be shown that gas phase<br />
halogen concentrations decrease. Chlorine concentrations<br />
decrease stronger than Bromine concentrations<br />
(∆Cl(tot):10-20%, ∆Br(tot):9%). For<br />
the aqueous phase we see an increase in bromine<br />
(∆HOBr:30%) and a decrease in chlorine concentrations<br />
(∆HOCl:40-50%). Overall aqueous phase<br />
chemistry is affected stronger than gas phase<br />
chemistry.<br />
Funding DFG-Emmy Noether Junior Research<br />
group Marhal GL 353/1-2<br />
Main publication Smoydzin & von Glasow<br />
[2006]