Basic Research Needs for Solar Energy Utilization - Office of ...
Basic Research Needs for Solar Energy Utilization - Office of ...
Basic Research Needs for Solar Energy Utilization - Office of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
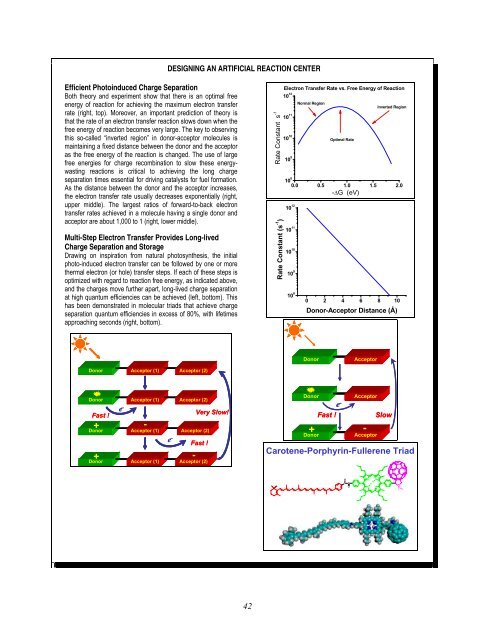
Efficient Photoinduced Charge Separation<br />
Both theory and experiment show that there is an optimal free<br />
energy <strong>of</strong> reaction <strong>for</strong> achieving the maximum electron transfer<br />
rate (right, top). Moreover, an important prediction <strong>of</strong> theory is<br />
that the rate <strong>of</strong> an electron transfer reaction slows down when the<br />
free energy <strong>of</strong> reaction becomes very large. The key to observing<br />
this so-called “inverted region” in donor-acceptor molecules is<br />
maintaining a fixed distance between the donor and the acceptor<br />
as the free energy <strong>of</strong> the reaction is changed. The use <strong>of</strong> large<br />
free energies <strong>for</strong> charge recombination to slow these energywasting<br />
reactions is critical to achieving the long charge<br />
separation times essential <strong>for</strong> driving catalysts <strong>for</strong> fuel <strong>for</strong>mation.<br />
As the distance between the donor and the acceptor increases,<br />
the electron transfer rate usually decreases exponentially (right,<br />
upper middle). The largest ratios <strong>of</strong> <strong>for</strong>ward-to-back electron<br />
transfer rates achieved in a molecule having a single donor and<br />
acceptor are about 1,000 to 1 (right, lower middle).<br />
Multi-Step Electron Transfer Provides Long-lived<br />
Charge Separation and Storage<br />
Drawing on inspiration from natural photosynthesis, the initial<br />
photo-induced electron transfer can be followed by one or more<br />
thermal electron (or hole) transfer steps. If each <strong>of</strong> these steps is<br />
optimized with regard to reaction free energy, as indicated above,<br />
and the charges move further apart, long-lived charge separation<br />
at high quantum efficiencies can be achieved (left, bottom). This<br />
has been demonstrated in molecular triads that achieve charge<br />
separation quantum efficiencies in excess <strong>of</strong> 80%, with lifetimes<br />
approaching seconds (right, bottom).<br />
Donor Acceptor (1)<br />
e- Donor Acceptor (1) Acceptor (2)<br />
Fast !<br />
e<br />
Very Slow!<br />
-<br />
Donor Acceptor (1) Acceptor (2)<br />
Fast !<br />
Very Slow!<br />
+<br />
-<br />
Donor Acceptor (1)<br />
+<br />
Donor Acceptor (1)<br />
DESIGNING AN ARTIFICIAL REACTION CENTER<br />
e- e- Acceptor (2)<br />
Acceptor (2)<br />
Fast !<br />
-<br />
Acceptor (2)<br />
42<br />
Rate Constant s -1<br />
Rate Constant (s -1 )<br />
Electron Transfer Rate vs. Free <strong>Energy</strong> <strong>of</strong> Reaction<br />
10 12<br />
10 11<br />
10 10<br />
10 9<br />
Normal Region<br />
Optimal Rate<br />
Inverted Region<br />
10<br />
0.0 0.5 1.0 1.5 2.0<br />
8<br />
-ΔG (eV)<br />
10 12<br />
10 11<br />
10 10<br />
10 9<br />
10 8<br />
0 2 4 6 8 10<br />
Donor-Acceptor Distance (Å)<br />
Donor Acceptor<br />
Donor Acceptor<br />
e- Donor Acceptor<br />
e- +<br />
Fast ! Slow<br />
-<br />
Donor Acceptor<br />
Carotene-Porphyrin-Fullerene Triad<br />
H<br />
N C<br />
O<br />
N N<br />
H<br />
N N<br />
H<br />
N<br />
CH3