- Page 1 and 2: 1National Academy of Sciences Natio
- Page 3 and 4: The Radiochemistry of Plutonium. Ge
- Page 5 and 6: PREFACE This report has been prepar
- Page 7 and 8: Procedures (Continued) CONTENTS (Co
- Page 9 and 10: IL GENEWL REVDZWS OF THE RADIWHEMIS
- Page 11 and 12: IV. CHEMISTRY OF PLUTONIUM OF SPECI
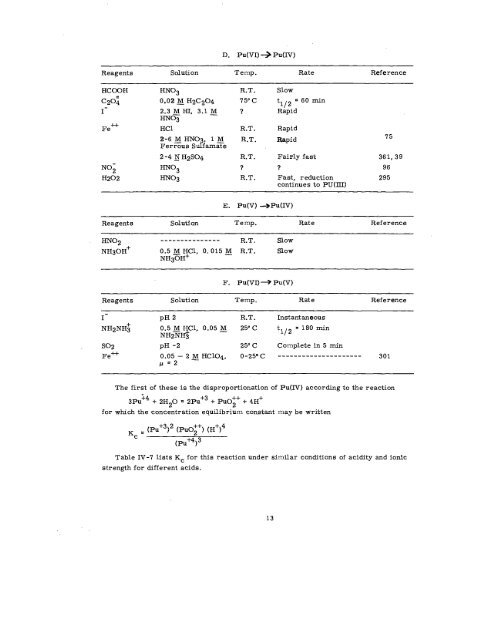
- Page 13 and 14: Solution TABLE IV-2. Behavior of PU
- Page 15 and 16: * Compiled primarily from the revie
- Page 17 and 18: TABLE IV-6. Oxidation-Reduction Rea
- Page 19: c.. Pu(IV)+ Pu(VI) Reagents Solutio
- Page 23 and 24: PU+4 has approximately the same ten
- Page 25 and 26: TABLE IV- 9. Ionic Potential of Plu
- Page 27 and 28: TABLE IV- 11. Stabflity Conetants o
- Page 29 and 30: TABLE IV- 12. Stability Constants o
- Page 31 and 32: D. 1 Co-precipitation and Precipita
- Page 33 and 34: is first oxidized to Pu(VI) with so
- Page 35 and 36: oxygen in the ratio 1:3. If an exce
- Page 37 and 38: the TBP cone entration. Solovkin 39
- Page 39 and 40: D, l? D Ic n. 0 5 10 Concentration
- Page 41 and 42: D 103 ~ ,.2 _ 10 I H ,0-1 ~ / P / P
- Page 43 and 44: 100( I0( 1( D 1.( 0. 0.0 0.00 Nltrl
- Page 45 and 46: Table IV- 16. (Continued) Extractan
- Page 47 and 48: Table IV-16. (Continued) Extr actan
- Page 49 and 50: In this equation, HA represents any
- Page 51 and 52: X[N05] = [HN03] ‘( ~ ~[N03] = HN0
- Page 53 and 54: Di-acidic compounds. Mono-2- ethylh
- Page 55 and 56: A may be either a simple anion or t
- Page 57 and 58: n 104 d Id 10 I 10-1 , a ,,81 0.1 M
- Page 59 and 60: The fact that Pu(III) does not extr
- Page 61 and 62: Fig. 25. Extraction of elements as
- Page 63 and 64: TABLE IV- 19. Distribution Coeffici
- Page 65 and 66: 10~ I ~ 0.01~ D 0.001 : 0,0001 . La
- Page 67 and 68: loi- 1“’’’’’’’”1
- Page 69 and 70: TABLE IV- 22. Extraction of Plutoni
- Page 71 and 72:
m * Amideb N, N-Dihexylformamide N,
- Page 73 and 74:
dependence on the TTA concentration
- Page 75 and 76:
1.0 D 0.1 —--L~,20 HNOO; CO&NT R
- Page 77 and 78:
cupferron, followed by back extract
- Page 79 and 80:
. more. Siekierski and Taube, 381 a
- Page 81 and 82:
TABLE IV- 27. Slope of the Dependen
- Page 83 and 84:
polymerization of the resin to prov
- Page 85 and 86:
co ; n 1000L z - 100 z o ~ m oL x z
- Page 87 and 88:
Volume distribution coefficient of
- Page 89 and 90:
affinity of several ions was Th(IV)
- Page 91 and 92:
95% of Pu(IV) was removed from a 0.
- Page 93 and 94:
2 d 1.0 I I I 1 I I 1 ,, , , I II r
- Page 95 and 96:
X“ 104 , 1 1 1 1 1 .— .-. I Ioa
- Page 97 and 98:
200 100 50 20 I O.10 ~ $ NPIX PUIIT
- Page 99 and 100:
Fig. 54. Adsorption of the elements
- Page 101 and 102:
Toribara et al. 403 used a liquid s
- Page 103 and 104:
V. DISSOLUTION OF PLUTON17JM SAMPLE
- Page 105 and 106:
m m TABLE VI- 30. Source Preparatio
- Page 107 and 108:
o 0 TAIIT.E VI-31. Techniques for M
- Page 109 and 110:
Solvent extraction of Pu into a liq
- Page 111 and 112:
TABLE VII-33. Basic Data for Critic
- Page 113 and 114:
Procedure No. 6. 7. 8. 9a, 9b , Aut
- Page 115 and 116:
Procedure 1. Determination of Pu in
- Page 117 and 118:
(e) (f) (g) (h) Where C v E The pre
- Page 119 and 120:
Procedure 2. Separation and determi
- Page 121 and 122:
Procedure 3. Separation and determi
- Page 123 and 124:
Procedure 4. Plutonium R. J. Morrow
- Page 125 and 126:
Procedure 5. Plutonium D. C, Hoffma
- Page 127 and 128:
Procedure m. Pipet tie samples (2~i
- Page 129 and 130:
Procedure 6. Separation of Plutoniu
- Page 131 and 132:
Procedure 7. Determination of Pu (R
- Page 133 and 134:
Procedure 8. Uranium and Plutonium
- Page 135 and 136:
15. Add 3 drops of HC1 and 1 drop o
- Page 137 and 138:
Procedure 9b. Separation of Plutoni
- Page 139 and 140:
Procedure 11. Uranium and Plutonium
- Page 141 and 142:
Procedure 12. Plutonium from Enviro
- Page 143 and 144:
9. 10. 11. 12. 13. 14. 15. 16. 17.
- Page 145 and 146:
10. 11. 12. 13. 14. 15. 16. 17. 16.
- Page 147 and 148:
Procedure 14. Separation of Plutoni
- Page 149 and 150:
Procedure 15. Separation of Pu befo
- Page 151 and 152:
Procedure 16. Separation of Plutoni
- Page 153 and 154:
Appendix (m) (n) (o) (P) Purificati
- Page 155 and 156:
Procedure 17. Separation of Np and
- Page 157 and 158:
Procedure 19. Determination of Plut
- Page 159 and 160:
NOTE: Reporting Results Results are
- Page 161 and 162:
Notes .. 1. 2. 3. The element prase
- Page 163 and 164:
3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13
- Page 165 and 166:
Procedure 22. Determination of Amer
- Page 167 and 168:
concentrated nitric acid in a block
- Page 169 and 170:
Preparation of Sample The 24-hr or
- Page 171 and 172:
Procedure 24. Determination of Plut
- Page 173 and 174:
Procedure 25. Determination of Plut
- Page 175 and 176:
“The Actinide Elements” (McGraw
- Page 177 and 178:
48. D. H. Boase, J. K. Foreman, and
- Page 179 and 180:
108. 109. 110. 111. 112. 113, 114.
- Page 181 and 182:
159. 160. 161. 162. 163. 164. 165.
- Page 183 and 184:
217. W. E. Keder, J. Inorg. Nucl. C
- Page 185 and 186:
279. 280. 281. 282. 283. 284. 285.
- Page 187 and 188:
339. 340. 341. 342. 343. 344. 345.
- Page 189 and 190:
397. 398. 399. 400. 401. 402. 403.
- Page 191 and 192:
451. C. E. Honey, Jr., R. N. R. Mul