THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
I.0<br />
D o. I<br />
K<br />
Pu (Vl)n Pu CO NCENTRAT10Nm2mg/ml<br />
o 1, ,, # “ , Oadju6tBd<br />
Pu(IV) ● Pu CONCENTRATION= 2 mg/ml<br />
w. II “ d.,, .<br />
,D<br />
. . . .<br />
aalustoa<br />
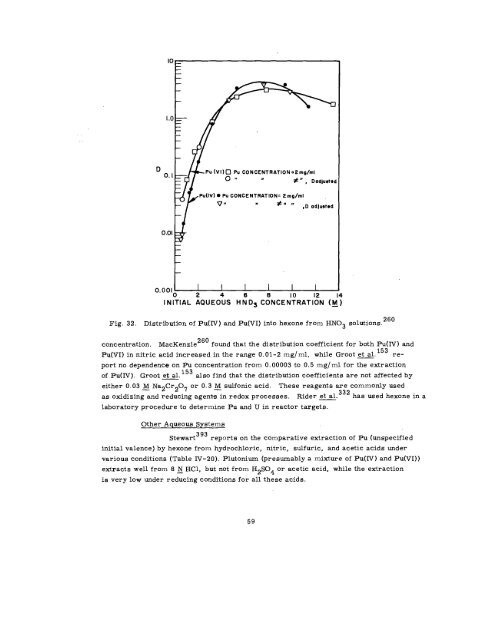
Fig. 32. Distribution of Pu(IV ) and Pu(VI) into hexone from HN03 solutions.<br />
concentration. MacKenzie<br />
260 found that the distribution coefficient for both Pu(IV) and<br />
Pu(VI) in nitric acid increased in the range 0,01-2 mg/ml, while Groot et al. 153 re-<br />
port no dependence on Pu concentration from 0.00003 to 0.5 mg/ml for the extraction<br />
153<br />
of Pu(IV). Groot et al. also find that the distribution coefficients are not affected by<br />
either (). 03 ~ Na2Cr207 or O.3 ~ 9ulfOniC acid. These reagents are commonly used<br />
as oxidizing and reducing agents in redox processes. Rider ~.332 has used hexone in a<br />
laboratory procedure to determine Pu and U in reactor targets.<br />
Other Aqueous Systems<br />
Stewart3’3 reports on the comparative extraction of Pu (unspecified<br />
initial valence) by hexone from hydrochloric, nitric, sulfuric, and acetic acids under<br />
various conditions (Table IV-20). Plutonium (presumably a mixture of Pu(IV) and Pu(VI))<br />
extracts well from 6 ~ HC1, but not from H2S04 or acetic acid, while the extraction<br />
is very low under reducing conditions for all these acids.<br />
59<br />
260