THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
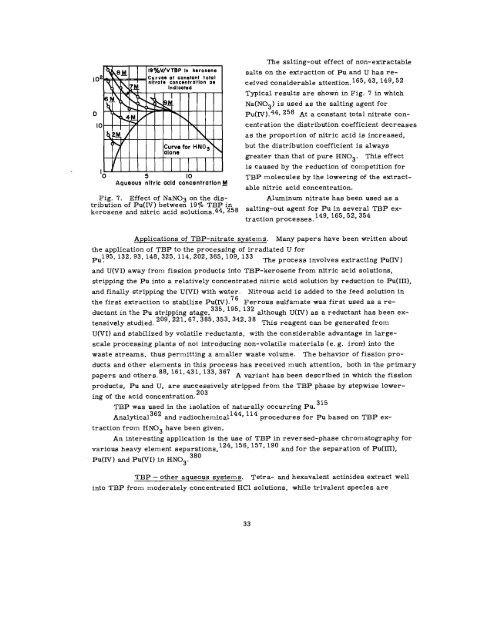
,02<br />
D<br />
10<br />
I<br />
‘o 5 10<br />
Aqueous nltrlc acid concentratlankJ<br />
Fig. 7. Effect of NaN03 on the distribution<br />
of Pu(IV) between lg~o TBP in<br />
kerosene and nitric acid solutions.44. 258<br />
The salting-out effect of non-extractable<br />
salts on the extraction of Pu and U has received<br />
considerable attention.<br />
165, 43, 149,52<br />
Typical results are shown in Fig. 7 in which<br />
Na(N03) is used as the salting agent for<br />
Pu(IV ). 44, 258 At a constant total nitrate con-<br />
centration the distribution coefficient decreases<br />
as the proportion of nitric acid is increased,<br />
but the distribution coefficient is always<br />
greater than that of pure HN03. This effect<br />
is caused by the reduction of competition for<br />
TBP molecules by the lowering of the extract-<br />
able nitric acid concentration.<br />
Aluminum nitrate has been used as a<br />
salting- out agent for Pu in several TBP extraction<br />
processes.<br />
149, 165, 52, 354<br />
Applications of TBP-nitrate systems. Many papers have been written about<br />
the application of TBP to the processing of irradiated U for<br />
PU195, 132, 93, 148,325, 114, 202,365, 109, 133<br />
The process involves extracting Pu(IV )<br />
and U(VI) away from fission products into TBP-kerosene from nitric acid solutions,<br />
stripping the Pu into a relatively concentrated nitric acid solution by reduction to Pu(IIf),<br />
and finally stripping the U(VI) with water. Nitrous acid is added to the feed solution in<br />
76<br />
the first extraction to stabilize Pu(IV). Ferrous sulfamate was first used as a re -<br />
ductant in the Pu stripping stage, 335, 195, 132 although U(IV) as a reductant has been extensively<br />
studied.<br />
209, 221, 67.385, 353, 342, 38<br />
This reagent can be generated from<br />
U(VI) and stabilized by volatile reductants, with the considerable advantage in large-<br />
scale processing plants of not introducing non-volatile materials (e. g. irorr) into the<br />
waste streams, thus permitting a smaller waste volume. The behavior of fission pro-<br />
ducts and other elements in this process has received much attention, both in the primary<br />
papers and others. 88,161,431,133,367<br />
A variant has been described in which the fission<br />
products, Pu and U, are successively stripped from the TBP phase by stepwise lowering<br />
of the acid concentration.<br />
203<br />
TBP was used in the isolation of naturally occurring Pu. 315<br />
Analytical<br />
362<br />
and radiochemical<br />
144, 114<br />
procedures for Pu based on TBP ex-<br />
traction from HN03 have been given.<br />
An interesting application is the use of TBP in reversed-phase chromatography for<br />
various heavy element separations, 124’ 156’157’190 and for the separation of Pu(IIT),<br />
Pu(IV) and Pu(VI) in HN03. 380<br />
TBP – other aqueous systems. Tetra- and hexavalent actinides extract well<br />
into TBP from moderately concentrated HC1 solutions, while trivalent species are<br />
33