THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
n2<br />
w<br />
:<br />
G<br />
.<br />
4<br />
3<br />
I<br />
c<br />
-1<br />
-2<br />
\<br />
\<br />
1 I<br />
z 4 6 8 10 12<br />
~ HCI<br />
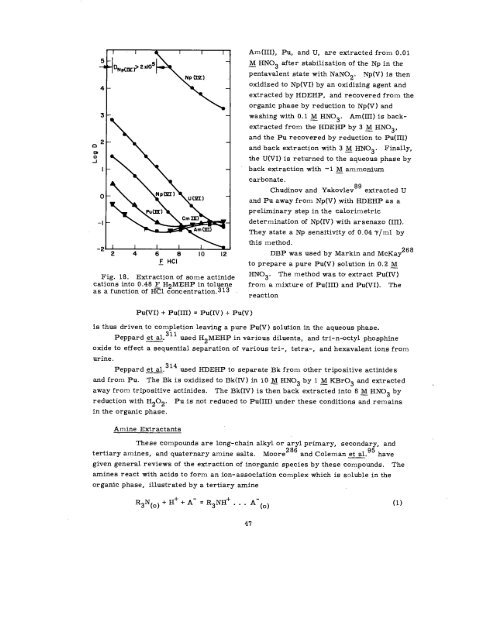
Fig. 18. Extraction of some actinide<br />
cations into 0.48 F H2MEHP in toluene<br />
as a function of H~l concentration.313<br />
Pu(VI) + Pu(IIf) = Pu(IV) + Pu(V)<br />
Am(III), Pu, and U, ax-e extracted from 0.01<br />
~ HN03 after stabilization of the Np in the<br />
pentavalent state with NaN02. Np(V ) is then<br />
oxidized to Np(VI) by an oxidizing agent and<br />
extracted by HDEHP, and recovered from the<br />
<strong>org</strong>anic phase by reduction to Np(V) and<br />
washing with O. 1 M HN03. Am(III) is back-<br />
—<br />
extracted from the HDEHP by 3 ~ HN03,<br />
and the Pu recovered by reduction to Pu(HI)<br />
and back extraction with 3 M HN03. Finally,<br />
—<br />
the U(VI) is returned to the aqueous phase by<br />
back extraction with -1 ~ ammonium<br />
carbonate.<br />
Chudinov and Yakovlev<br />
89 extracted U<br />
and Pu away from Ifp(V ) with HDEHP as a<br />
preliminary step in the calorimetric<br />
determination of Np(lY ) with arsenazo (Il_f).<br />
They state a Np sensitivity y of 0.04 ?/ml by<br />
this method.<br />
DBP was used by Markin and McKay 268<br />
to prepare a pure Pu(V) solution in 0.2<br />
—<br />
M<br />
HN03 . The method was ta extract Pu(IV )<br />
from a mixture of Pu(IIf) and Pu(VI). The<br />
reaction<br />
is thus driven to completion leaving a pure Pu(V) solution in the aqueous phase.<br />
Peppard et al. 311 used H MEHP in various diluents, and tri-n-octyl phosphine<br />
2<br />
oxide to effect a sequential separation of various tri-, tetra-, and hexavalent ions from<br />
urine.<br />
Peppard et al. 314 used HDEHP to separate Bk from other tripositive actinides<br />
and from Pu. The Bk is oxidized to Bk(lY) in 10 M HN03 by 1 M KBr03 and extracted<br />
— —<br />
away from tripositive actinides. The Bk(lY) is then back extracted into 8 ~ HN03 by<br />
‘eduction ‘ith ‘2°2 “<br />
in the <strong>org</strong>anic phase.<br />
Amine Extractants<br />
Pu is not reduced to Pu(III) under these conditions and remains<br />
These compounds are long- chain alkyl or aryl primary, secondary, and<br />
tertiary amines, and quaternary amine salts. Moore286 and Coleman<br />
95<br />
et al. have<br />
given general reviews of the extraction of in<strong>org</strong>anic species by these compounds. The<br />
amines react with acids to form an ion-association complex which is soluble in the<br />
<strong>org</strong>anic phase, illustrated by a tertiary amine<br />
R3N(0)<br />
+H++A-=R3NH+...A-<br />
47<br />
(0)<br />
(1)