THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
D<br />
103 ~<br />
,.2 _<br />
10<br />
I<br />
H<br />
,0-1 ~<br />
/<br />
P<br />
/<br />
Pu(m)<br />
/pU(m)<br />
j’ >U(m)<br />
Pu (m)<br />
AQUEOUS I-& CON:ENTR:TION ([j)<br />
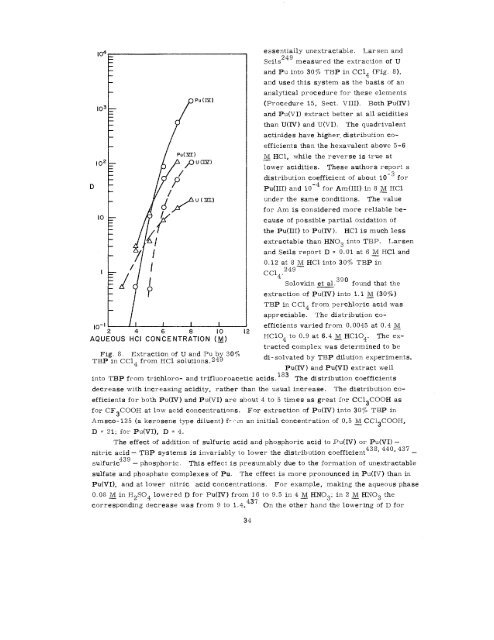
Fig. 8. Extraction of U and Pub<br />
TBP in CC14 from HC1 solutions .24 $3070<br />
essentially unextractable. Larsen and<br />
Seils249 measured the extraction of U<br />
and Pu into 30% TEIP in CC14 (Fig. 8),<br />
and used this system as the basis of an<br />
analytical procedure for these elements<br />
(Procedure 15, Sect. VIII). Both Pu(IV)<br />
and Pu(VI) extract better at all acidities<br />
than U(IV) and “U(VI). The quadrivalent<br />
actinides have higher, distribution co-<br />
efficients than the hexavalent above 5-6<br />
~ HC1, while the reverse is true at<br />
lower acidities. These authors report a<br />
distribution coefficient of about 10 -3 for<br />
Pu(III) and 10-4 for Am(III) in 8 ~ HC1<br />
under the same conditions. The value<br />
for Am is considered more reliable be-<br />
cause of possible partial oxidation of<br />
the Pu(III) to Pu(IV). HC1 is much less<br />
extractable than HN03 into TBP. Larsen<br />
and Seils report D = 0.01 at 6 M HC1 and<br />
—<br />
0.12 at 8&l HC1 into 3070 TBP in<br />
CC14.2’4’<br />
Solovkin et al. 390 found that the<br />
extraction of Pu(IV) into 1.1 &l (3070)<br />
TBP in CC14 from perchloric acid was<br />
appreciable. The distribution co-<br />
efficients varied from 0.0045 at 0.4 M .<br />
HC104 to 0.9 at 6.4 ~ HC104. The ex-<br />
tracted complex was determined to be<br />
di-solvated by TBP dilution experiments.<br />
Pu(IV) and Pu(VI) extract well<br />
into TBP from trichloro - and trifluoroacetic acids. 183 The distribution coefficients<br />
decrease with increasing acidity, rather than the usual increase. The distribution co-<br />
efficients for both Pu(IV) and Pu(VI) are about 4 to 5 times as great for CC13COOH as<br />
for CF3COOH at low acid concentrations. For extraction of Pu(IV) into 30~0 TBP in<br />
Amsco- 125 (a kerosene type diluent) f> m an initial concentration of 0.5 M CC13COOH,<br />
—<br />
D = 21; for Pu(VI), D = 4.<br />
The effect of addition of sulfuric acid and phosphoric acid to l%(~) or Pu(VI) –<br />
nitric acid — TE3P systems is invariably to lower the distribution coefficient<br />
438, 440>437<br />
sulfuric439<br />
– phosphoric. This effect is presumably due to the formation of unextractable<br />
sulfate and phosphate complexes of Pu. The effect is more pronounced in Pu(IV) than in<br />
Pu(VI), and at lower nitric acid concentrations. For example, making the aqueous phase<br />
0.08 M in H2S04 lowered D for Pu(IY) from 16 to 9.5 in 4 JYJHN03; in 2 ~ HN03 the<br />
437<br />
corresponding decrease was from 9 to 1.4. On the other hand the lowering of D for<br />
34<br />
—