THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Precipitation<br />
Precipitation of macro quantities of Pu is necessary in many analytical<br />
and radiochemical procedures. The precipitation reactions which have been found<br />
useful in practice will be reviewed in this section. The use of various precipitates as<br />
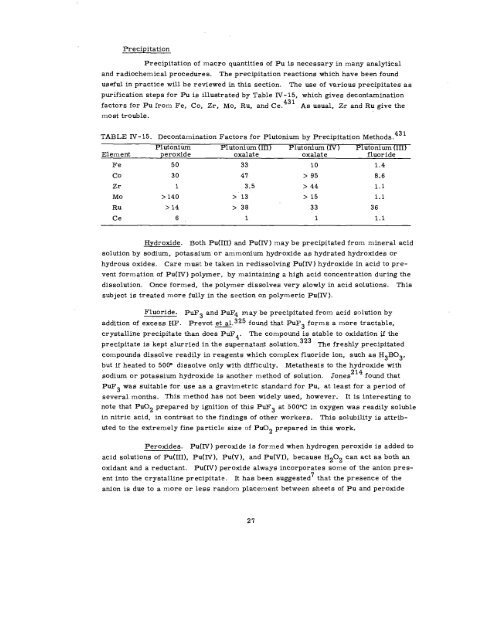
purification steps for Pu is illustrated by Table IV- 15, which gives decontamination<br />
factors for Pu from Fe, Co, Zr, Mo, Ru, and Ce. 431 As usual, Zr and Ru give the<br />
most trouble.<br />
TABLE IY - 15. Decontamination Factors for Plutonium by Precipitation Methods. 431<br />
Plutonium Plutonium (IH) Plutonium (IV) Plutonium (Hf)<br />
Element peroxide oxalate oxalate fluoride<br />
Fe 50 33 10 1.4<br />
co 30 47 > 95 8.6<br />
Zr 1 3.5 >44 1.1<br />
Mo >140 > 13 > 15 1.1<br />
Ru >14 > 38 33 36<br />
Ce 6, 1 1 1.1<br />
Hydroxide. Both Pu(IIZ) and Pu(IY ) may be precipitated from mineral acid<br />
solution by sodium, potassium or ammonium hydroxide as hydrated hydroxides or<br />
hydrous oxides. Care must be taken in redissolving Pu(I17) hydroxide in acid to pre-<br />
vent formation of Pu(IY) polymer, by maintaining a high acid concentration during the<br />
dissolution. Once formed, the polymer dissolves very slowly in acid solutions. This<br />
subject is treated more fully in the section on polymeric Pu(IV).<br />
Fluoride. PuF3 and PuF4 may be precipitated from acid solution by<br />
addition of excess HF. Prevot et al. 325 found that PuF3 forms a more tractable,<br />
crystalline precipitate than does PuF4. The compound is stable to oxidation if the<br />
323<br />
precipitate is kept slurried in the supernatant solution. The freshly precipitated<br />
compounds dissolve readily in reagents which complex fluoride ion, such as H3B03,<br />
but if heated to 500” dissolve only with difficulty. Metathesis to the hydroxide with<br />
sodium or potassium hydroxide is another method of solution. Jones214 found that<br />
PuF3 was suitable for use as a gravimetric standard for Pu, at least for a period of<br />
several months. This method has not been widely used, however. It is interesthg to<br />
note that PU02 prepared by ignition of this PuF3 at 500”C in oxygen was readily soluble<br />
in nitric acid, in contrast to the findings of other workers. This volubility is attrib-<br />
uted to the extremely fine particle size of PU02 prepared in this work.<br />
Peroxides. Pu(IV) peroxide is formed when hydrogen peroxide is added to<br />
acid solutions of PU(III)J Pu(IV), Pu(V), and Pu(VI), because H202 can act as both an<br />
oxidant and a reductant. Pu(IV) peroxide always incorporates some of the anion pres-<br />
ent into the crystalline precipitate. It has been suggested7 that the presence of the<br />
anion is due to a more or less random placement between sheets of Pu and peroxide<br />
27