THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
a<br />
ml<br />
0<br />
-1<br />
5<br />
0<br />
4<br />
3<br />
2<br />
-1<br />
-2<br />
-3<br />
~- x<br />
I I<br />
\<br />
\<br />
I<br />
-1 0 1,<br />
Log HN03 CONCENTRATION w)<br />
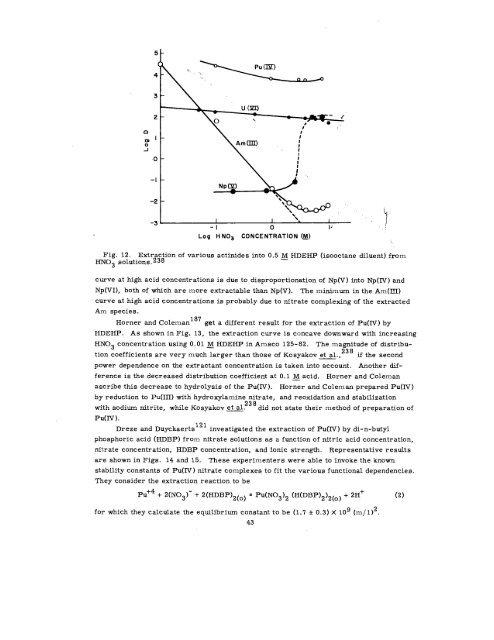
Fig. 12. Extraction of various actinides into 0.5<br />
—<br />
M HDEHP (isooctane diluent) from<br />
HN03 solutions.238<br />
curve at high acid concentrations is due to disproportionation of Np(V ) into Np(lY ) and<br />
Np(VI), both of which are more extractable than Np(V). The minimum in the Am(lII)<br />
curve at high acid concentrations is probably due to nitrate completing of the extracted<br />
Am species.<br />
Horner and Coleman 187 get a different result for the extraction of Pu(lV ) by<br />
HDEHP. As shown in Fig. 13, the extraction curve is concave downward with increasing<br />
HN03 concentration using 0.01 ~ HDEHP in Amsco 125-82. The magnitude of distribution<br />
coefficients are very much larger than those of Kosyakov ~.,<br />
238 ~ the second<br />
power dependence on the extractant concentration is taken into account. Another dif-<br />
ference is the decreased distribution coefficient at 0.1 ~ acid. Homer and Coleman<br />
ascribe this decrease to hydrolysis of the Pu(IV). Horner and Coleman prepared Pu(IY)<br />
by reduction to Pu(IIf) with hydroxylamine nitrate, and reoxidation and stabilization<br />
238<br />
with sodium nitrite, while Kosyakov et al. dld not state their method of preparation of<br />
Pu(lv).<br />
Dreze and Duyckaerts<br />
\<br />
121 investigated the extraction of Pu(IY) by di - n-butyl<br />
phosphoric acid (HDBP) from nitrate solutions as a function of nitric acid concentration,<br />
nitrate concentration, HDBP concentration, and ionic strength. Representative results<br />
are shown in Figs. 14 and 15. These experimenters were able to invoke the known<br />
stability constants of Pu(IV ) nitrate complexes to fit the various fwctional dependencies.<br />
They consider the extraction reaction to be<br />
PU+4 + 2(N03 )- + 2(HDBP)2(0) - PU(N03)2 (H(DBP)2)2(0) + 2H+ (2)<br />
for which they calculate the equilibrium constant to be (1.7 + 0.3) X 109 (m/1 )2.<br />
43<br />
I