THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
m<br />
*<br />
Amideb<br />
N, N-Dihexylformamide<br />
N, N- Dibut ylacetamide<br />
N, N-Dibutylpropionamide<br />
N, N-Dibutylisobuty ramide<br />
N, N-Dibutylpivalamide<br />
N, N-Dibutylbutyramide<br />
N, N-Di-isobutylbutyramide<br />
N, N-Di-isobutylisobutyramide<br />
N, N- Dicycloheqlformamide<br />
N, N-Dicyclohexylacetamide<br />
N, N-Dicyclohexylbutyramide<br />
N, N- Dibut yl - 2- ethylhexanamide<br />
N, N-Dimethyldecanamide<br />
N, N-Diethyldecanamide<br />
1- Hexanoylpiperidine<br />
1-(2 -Ethylhexanoyl) - piperidine<br />
N, N-Di-sec-butylhexanamide<br />
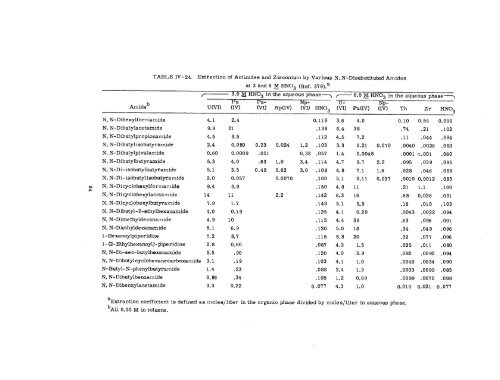
TABLE IV- 24. Extraction of Actinides and Zirconium by Various N, N-Disubstituted Amides<br />
at 3 and 6 ~ HN03 (Ref. 379), a<br />
~— 3.0 ~ HN03 in the aqueous phase ~ ~ 6.0 ~ HN03 in the aqueous phase=<br />
P 1+ N<br />
u (w) (1:) (~~ NP(IV) (1% HN03 ~(VI) Pu(IV) (1$~ Th Zr HN03<br />
14<br />
4.1<br />
9.9<br />
4.5<br />
2.4<br />
0.60<br />
5.3<br />
5.1<br />
2,0<br />
9.4<br />
7.9<br />
4.0<br />
4.9<br />
5.1<br />
7.2<br />
2,8<br />
5.5<br />
N, N- Dibutylcyclohexane carboxamide 3.1<br />
N-But yl- N-phenylbutyramide 1.4<br />
N, N-Dibutylbenzamide 0.86<br />
N, N-Dibenzylacetamide 3.3<br />
21<br />
11<br />
10<br />
2.4<br />
3.5<br />
0.080 0.23<br />
0.0009 .051<br />
4.0 .63<br />
3.5 0.48<br />
0.057<br />
9.9<br />
1.7<br />
0.19<br />
6.9<br />
8.7<br />
0.60<br />
.90<br />
.19<br />
,23<br />
.34<br />
0.22<br />
0.119<br />
,138<br />
.112<br />
0.024 1.2 .103<br />
0.33 .057<br />
1.0 3,4 .114<br />
0.62 3.0 .108<br />
0.0070 .100<br />
.150<br />
2.2 .142<br />
.148<br />
.125<br />
.115<br />
.120<br />
.115<br />
.087<br />
.120<br />
.103<br />
.086<br />
.105<br />
0.077<br />
3.6<br />
6.4<br />
4.5<br />
3.3<br />
1.4<br />
4.7<br />
4.8<br />
3.1<br />
4.8<br />
6.3<br />
5.1<br />
4.1<br />
4.4<br />
5.0<br />
5.6<br />
4,2<br />
4.0<br />
4.1<br />
2.4<br />
1.2<br />
4.3<br />
38<br />
11<br />
16<br />
4.0<br />
7.2<br />
0.21 0.070<br />
0.0046<br />
8.7 2.2<br />
7.1 1.6<br />
0.11 0.037<br />
aExtraction coefficient is defined as moles/liter in the <strong>org</strong>anic phase divided by moles/liter in aqueous phase.<br />
.<br />
“All 0.50 M in toluene.<br />
39<br />
16<br />
20<br />
5.9<br />
0.29<br />
1.5<br />
3.9<br />
1.0<br />
1.,3<br />
0.69<br />
1.0<br />
0.10 0.54 0.090<br />
,74 .21 .102<br />
.11 .044 .094<br />
.0040 .0026 .083<br />
.0001