THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1.0<br />
10-1<br />
D<br />
,.-2<br />
!<br />
/<br />
//<br />
/<br />
I<br />
* -<br />
/3<br />
4 / I<br />
i<br />
,<br />
I<br />
!<br />
/<br />
i<br />
I<br />
/<br />
!<br />
;<br />
/<br />
,1<br />
1 / /<br />
I<br />
r<br />
/2<br />
‘1.2 and3<br />
I<br />
— I PUUII)<br />
--2 0.005 MFe(ll) sulphOm GtO(+ 0.006<br />
M hydr,oxylamlne)<br />
-.-3 0.005-o.02M Fe OTl+hydrazlne;<br />
-..-4 0.005 -0.04Mhydroxyla mine.<br />
/ 1<br />
1<br />
M(HN03)\20<br />
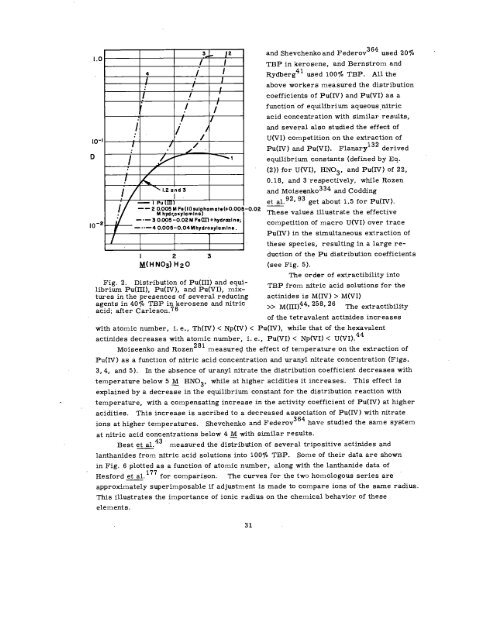
Fig. 2. Distribution of Pu(III) and equilibrium<br />
Pu(lII), Pu(IV), and Pu(VI), mixtures<br />
in the presences of several reducing<br />
agents in 40~0 TBP in kerosene and nitric<br />
acid; after Carleson.76<br />
3<br />
/<br />
-0.02<br />
364<br />
and Shevchenko and Federov used 20’7.<br />
TBP in kerosene, and Bernstrom and<br />
Rydberg 41 used 1007. TBP. All the<br />
above workers measured the distribution<br />
coefficients of Pu(IV ) and Pu(VI) as a<br />
function of equilibrium aqueous, nitric<br />
acid concentration with similar results,<br />
and several also studied the effect of<br />
U(VI) competition on the extraction of<br />
Pu(IV ) and Pu(VI). Flanary<br />
132<br />
derived<br />
equilibrium constants (defined by Eq.<br />
(2)) for U(VI), HN03, and Pu(IY) of 22,<br />
0.18, and 3 respectively, while Rozen<br />
and Moiseenko334 and Codding<br />
et al 92, 93<br />
—“<br />
get about 1.5 for Pu(IY).<br />
These values illustrate the effective<br />
competition of macro U(VI) over trace<br />
Pu(IV ) in the simultaneous extraction of<br />
these species, resulting in a large re-<br />
duction of the Pu distribution coefficients<br />
(see Fig. 5).<br />
The order of extractability into<br />
TBP from nitric acid solutions for the<br />
actinides is M(IV) > M(VI)<br />
>> M(III)?4’ 258’26 The extractability<br />
of the tetravalent actinides increases<br />
with atomic number, i. e., Th(lY) < Np(lY) < Pu(IV), while that of the hexavalent<br />
actinides decreases with atomic number, i. e., Pu(VI) < Np(VI) < U(VI). 44<br />
Moiseenko and Rozen 281 measured the effect of temperature on the extraction of<br />
Pu(lV) as a function of nitric acid concentration and uranyl nitrate concentration (Figs.<br />
3, 4, and 5). In the absence of uranyl nitrate the distribution coefficient decreases with<br />
temperature below 5 ~ HN03, while at higher acidities it increases. This effect is<br />
explained by a decrease in the equilibrium constant for the distribution reaction with<br />
temperature, with a compensating increase in the activity coefficient of Pu(IY) at higher<br />
acidities. This increase is ascribed to a decreased as:j:iation of Pu(IW) with nitrate<br />
ions at higher temperatures. Shevchenko and Federov have studied the same system<br />
at nitric acid concentrations below 4 ~ with similar results.<br />
Best e~.43 measured the distribution of several tripo sitive actinides and<br />
lanthanides from nitric acid solutions into 1007. TBP. Some of their data are shown<br />
in Fig. 6 plotted as a function of atomic number, along with the lanthanide data of<br />
Hesford ~. ’77 for comparison. The curves for the two homologous series are<br />
approximately superimposable if adjustment is made to compare ions of the same radius<br />
This illustrates the importance of ionic radius on the chemical behavior of these<br />
elements.<br />
31