THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
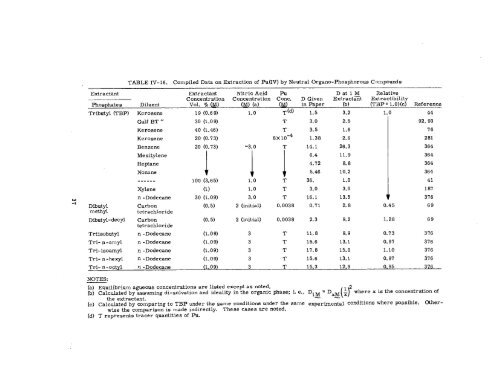
TABLE IV- 16. Compiled Data on Extraction of Pu(IV) by Neutral ~rgano-phosphorous Compounds<br />
Extractant Extractant Nitric Acid Pu Datl M Relative<br />
Concentration Concentration Cone. D Given Extracta~t Extractability<br />
Phosphatea<br />
Tribut yl (TBP)<br />
Ililuent<br />
Kerosene<br />
Vol. Y, (M)<br />
19 (0.69)<br />
(M) (a)<br />
1.0<br />
(M)<br />
, –.<br />
T(d)<br />
in Paper<br />
1.5<br />
(b)<br />
3.2<br />
(TBP = I. O)(c)<br />
1.0<br />
Reference<br />
44<br />
Gulf BT “<br />
Kerosene<br />
Kcroaene<br />
Benzene<br />
Mesitylene<br />
Heptane<br />
Nonane<br />
------<br />
Xylene<br />
n -Dodecane<br />
Dibut yl Carbon<br />
methyl tctrachloride<br />
Dibutyl - decyl Carbon<br />
tetrachloride<br />
Triisobutyl n -Dodecane<br />
Tri- n-amyl n -Dodecane<br />
Tri-iaoamyl n -Dode cane<br />
Tri- n -hexyl n -Dodecane<br />
30 (1.09)<br />
40 (1.46)<br />
20 (0.73)<br />
20 (0.73)<br />
100 (3,65)<br />
(1)<br />
30 (1.09)<br />
(o. 5)<br />
(o. 5)<br />
(1.09)<br />
(1.09)<br />
(1.09)<br />
(1.09)<br />
-3.0<br />
1.0<br />
1.0<br />
3.0<br />
2 (initial)<br />
2 (initial)<br />
3<br />
3<br />
3<br />
3<br />
T<br />
T<br />
.9X1 O-4<br />
Tri- n -octyl n -Dodccane (1,09) 3 T 15.3 12.9 0.95 376<br />
NOTES:<br />
T<br />
T<br />
T<br />
0.0038<br />
0.0036<br />
T<br />
T<br />
T<br />
T<br />
3.0<br />
3.5<br />
1.36<br />
14.1<br />
6.4<br />
4.72<br />
5.46<br />
36.<br />
3.0<br />
16.1<br />
0.71<br />
2.3<br />
11.8<br />
15.6<br />
17.8<br />
15.6<br />
2.5<br />
1.6<br />
2.6<br />
26.3<br />
11.9<br />
8.8<br />
10.2<br />
1,0<br />
3.0<br />
13.5<br />
2.8<br />
9.2<br />
9.9<br />
13.1<br />
15.0<br />
13.1<br />
92, 93<br />
76<br />
281<br />
I 0.45 69<br />
364<br />
364<br />
364<br />
364<br />
41<br />
187<br />
376<br />
1.26 69<br />
0.73 376<br />
0.97 376<br />
1.10 376<br />
0.97 376<br />
(a) Equilibrium agueous concentration are listed except as noted.<br />
(b) Calculated by aaaurning<br />
the extractant.<br />
di- solvation and ideality in the <strong>org</strong>anic phaae; i. e., D1 ~ = Dfl ~ 2 where<br />
()<br />
x is the concentration of<br />
(c) Calculated by comparing to TBP under the same conditions under the same ev~rime~al conditions where poaaible. Otherwise<br />
the comparison is made indirectly. These caaea are noted.<br />
(d) T representa tracer quantities of Pu.