THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
the TBP cone entration. Solovkin 391 has calculated distribution coefficients for this<br />
system on a semi-empirical basis and obtained good agreement with experimental data.<br />
Tetra- and hexavalent Pu as well as other actinides have been shown to be di-<br />
solvates in the <strong>org</strong>anic phase. 44 The extracted complexes are then PU(N03)4 r 2 TBP<br />
for Pu(IV) and PU02(N03)2 . 2 TBP for Pu(VI). Work with trivalent Pu has shown that<br />
the extracted complex is tri-solvated, Pu(N03)3 3 TBP. 372 Laxminarayanan et al. 252<br />
have shown that Pu(IV) in 2-4 ~ HTT03 is associated with an average of 2.6 nitrate ions<br />
and does not exist as undissociated Pu(N03)4 by combining solvent extraction data<br />
from several solvents. There is direct evidence that the complexes are un-ionized in<br />
the <strong>org</strong>anic phase. 170<br />
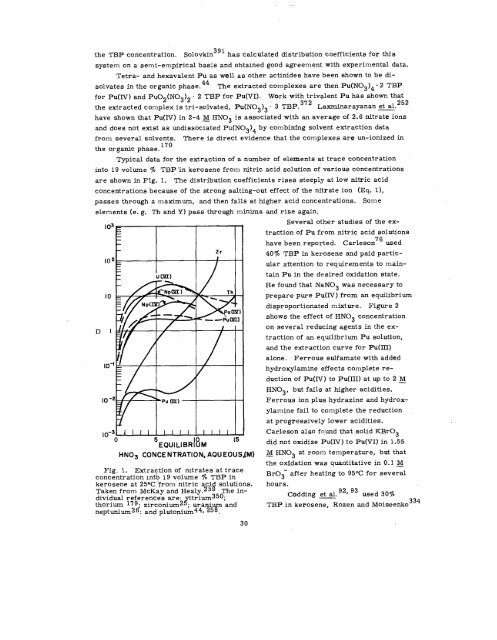
Typical data for the extraction of a number of elements at trace concentration<br />
into 19 volume ~. TBP ‘in kerosene from nitric acid solution of various concentrations<br />
are shown in Fig. 1. The distribution coefficients rises steeply at low nitric acid<br />
concentrations because of the strong salting-out effect of the nitrate ion (Eq. 1),<br />
passes through a maximum, and then falls at higher acid concentrations. Some<br />
elements (e. g. Th and Y) pass through minima and rise again.<br />
,o-’o&u+dLu+<br />
I I I<br />
I Zr I<br />
E(XJl LlBRi8M<br />
HN03 CONCENTRATION, AQUEOUSJM)<br />
Fig. 1, Extraction of mtrates at trace<br />
concentration mt~ 19 volume ~0 TBP in<br />
kerosene at 25oC from nitric aci 258 solutions<br />
Taken from McKay and Healy. The in- -<br />
dividual reference’s are: ~trium350;<br />
thorium 179. zirconium2 , uranium and<br />
neptunium2d: and plutonium44~ 258.<br />
30<br />
Several other studies of the ex-<br />
traction of Pu from nitric acid solutions<br />
have been reported. Carleson<br />
76<br />
used<br />
40~. TBP in kerosene and paid partic-<br />
ular attention to requirements to main-<br />
tain Pu in the desired oxidation state.<br />
He found that NaN03 was necessary to<br />
prepare pure Pu(IY) from an equilibrium<br />
disproportionated mixture. Figure 2<br />
shows the effect of HN03 concentration<br />
on several reducing agents in the ex-<br />
traction of an equilibrium Pu solution,<br />
and the extraction curve for Pu(llI)<br />
alone. Ferrous sulfamate with added<br />
hydroxylamine effects complete re-<br />
duction of Pu(IV ) to Pu(IH) at up to 2 ~<br />
HN03, but fails at higher acidities.<br />
Ferrous ion plus hydrazine and hydrox-<br />
ylamine fail to complete the reduction<br />
at progressively lower acidities.<br />
Carleson also found that solid KBr03<br />
did not oxidize Pu(IV) to Pu(VI) in 1.55<br />
~ HN03 at room temperature, but that<br />
the oxidation was quantitative in 0.1 ~<br />
Br03- after heating to 95°C for several<br />
hours.<br />
Codding et al. 92, 93 used 307.<br />
TBP in kerosene, Rozen and Moiseenko<br />
334