THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
THE RADIOCHEMISTRY OF PLUTONIUM - Sciencemadness.org
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
In this equation, HA represents any monoacidic ester of phosphoric acid.<br />
This equation has been shown to be correct for di- and trivalent ions, but tetra-<br />
valent ions in general show a more complex behavior. The extracted complex is some-<br />
times further solvated in the <strong>org</strong>anic phase and nitrate, chloride, and even perchlorate<br />
complexes may be involved in the extraction reaction, depending on the specific aqueous<br />
conditions employed. Thus, no general reaction can be proposed which will account<br />
for all of the observed behavior.<br />
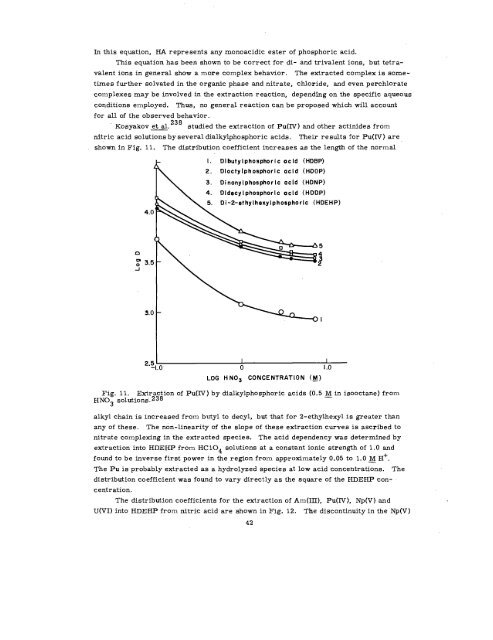
Kosyakov et al. 238 studied the extraction of Pu(IV) and other actinides from<br />
nitric acid solutions by several dialkylphosphoric acids. Their results for Pu(IV) are<br />
shown in Fig. 11. The distribution coefficient increases as the length of the normal<br />
a<br />
4.0<br />
: 3.5<br />
J<br />
3.0<br />
2.5<br />
\<br />
1. Dlbutyl phosphoric acid (HDBP)<br />
2. Dloctylphosphoric acid (HOOP)<br />
3. Dinonylphogpharlc acid (HDNP)<br />
4. Dldecylphosphorlc acid (HDDP)<br />
5. Di-2-ethylhexyl phosphoric (HDEHP)<br />
I I<br />
) o I.0<br />
LOG HN03 CONCENTRATION (~)<br />
Fig. 11. Extraction of Pu(IV) by dialkylphosphoric acids (0.5 ~ in isooctane) from<br />
HN03 solutions’. 238<br />
alkyl chain is increased from butyl to decyl, but that for 2-ethylhexyl is greater than<br />
any of these. The non-linearity of the slope of these extraction curves is ascribed to<br />
nitrate completing in the extracted species. The acid dependency was determined by<br />
extraction into HDEHP from HC 104 solutions at a constant ionic strength of 1.0 and<br />
found to be inverse first power in the region from approximately 0.05 to 1.0 ~ H+.<br />
The Pu is probably extracted as a hydrolyzed species at low acid concentrations. The<br />
distribution coefficient was found to vary directly as the square of the HDEHP con-<br />
centration.<br />
The distribution coefficients for the extraction of Am(III), Pu(IV ), Np(V ) and<br />
U(VI) into HDEHP from nitric acid are ahown in Fig. 12. The discontinuity y in the NP(V )<br />
42