Donepezil, rivastigmine, galantamine and memantine for ...
Donepezil, rivastigmine, galantamine and memantine for ...
Donepezil, rivastigmine, galantamine and memantine for ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
14<br />
Clinical effectiveness<br />
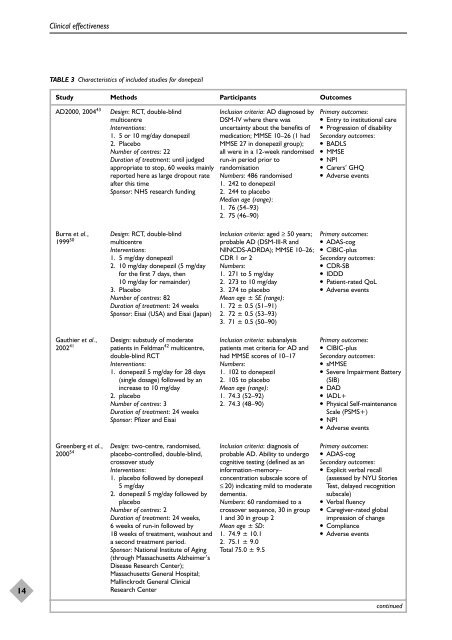
TABLE 3 Characteristics of included studies <strong>for</strong> donepezil<br />
Study Methods Participants Outcomes<br />
AD2000, 2004 43 Design: RCT, double-blind<br />
multicentre<br />
Interventions:<br />
1. 5 or 10 mg/day donepezil<br />
2. Placebo<br />
Number of centres: 22<br />
Duration of treatment: until judged<br />
appropriate to stop, 60 weeks mainly<br />
reported here as large dropout rate<br />
after this time<br />
Sponsor: NHS research funding<br />
Burns et al.,<br />
1999 50<br />
Gauthier et al.,<br />
2002 41<br />
Greenberg et al.,<br />
2000 54<br />
Design: RCT, double-blind<br />
multicentre<br />
Interventions:<br />
1. 5 mg/day donepezil<br />
2. 10 mg/day donepezil (5 mg/day<br />
<strong>for</strong> the first 7 days, then<br />
10 mg/day <strong>for</strong> remainder)<br />
3. Placebo<br />
Number of centres: 82<br />
Duration of treatment: 24 weeks<br />
Sponsor: Eisai (USA) <strong>and</strong> Eisai (Japan)<br />
Design: substudy of moderate<br />
patients in Feldman 42 multicentre,<br />
double-blind RCT<br />
Interventions:<br />
1. donepezil 5 mg/day <strong>for</strong> 28 days<br />
(single dosage) followed by an<br />
increase to 10 mg/day<br />
2. placebo<br />
Number of centres: 3<br />
Duration of treatment: 24 weeks<br />
Sponsor: Pfizer <strong>and</strong> Eisai<br />
Design: two-centre, r<strong>and</strong>omised,<br />
placebo-controlled, double-blind,<br />
crossover study<br />
Interventions:<br />
1. placebo followed by donepezil<br />
5 mg/day<br />
2. donepezil 5 mg/day followed by<br />
placebo<br />
Number of centres: 2<br />
Duration of treatment: 24 weeks,<br />
6 weeks of run-in followed by<br />
18 weeks of treatment, washout <strong>and</strong><br />
a second treatment period.<br />
Sponsor: National Institute of Aging<br />
(through Massachusetts Alzheimer’s<br />
Disease Research Center);<br />
Massachusetts General Hospital;<br />
Mallinckrodt General Clinical<br />
Research Center<br />
Inclusion criteria: AD diagnosed by<br />
DSM-IV where there was<br />
uncertainty about the benefits of<br />
medication; MMSE 10–26 (1 had<br />
MMSE 27 in donepezil group);<br />
all were in a 12-week r<strong>and</strong>omised<br />
run-in period prior to<br />
r<strong>and</strong>omisation<br />
Numbers: 486 r<strong>and</strong>omised<br />
1. 242 to donepezil<br />
2. 244 to placebo<br />
Median age (range):<br />
1. 76 (54–93)<br />
2. 75 (46–90)<br />
Inclusion criteria: aged ≥ 50 years;<br />
probable AD (DSM-III-R <strong>and</strong><br />
NINCDS-ADRDA); MMSE 10–26;<br />
CDR 1 or 2<br />
Numbers:<br />
1. 271 to 5 mg/day<br />
2. 273 to 10 mg/day<br />
3. 274 to placebo<br />
Mean age ± SE (range):<br />
1. 72 ± 0.5 (51–91)<br />
2. 72 ± 0.5 (53–93)<br />
3. 71 ± 0.5 (50–90)<br />
Inclusion criteria: subanalysis<br />
patients met criteria <strong>for</strong> AD <strong>and</strong><br />
had MMSE scores of 10–17<br />
Numbers:<br />
1. 102 to donepezil<br />
2. 105 to placebo<br />
Mean age (range):<br />
1. 74.3 (52–92)<br />
2. 74.3 (48–90)<br />
Inclusion criteria: diagnosis of<br />
probable AD. Ability to undergo<br />
cognitive testing (defined as an<br />
in<strong>for</strong>mation–memory–<br />
concentration subscale score of<br />
≤ 20) indicating mild to moderate<br />
dementia.<br />
Numbers: 60 r<strong>and</strong>omised to a<br />
crossover sequence, 30 in group<br />
1 <strong>and</strong> 30 in group 2<br />
Mean age ± SD:<br />
1. 74.9 ± 10.1<br />
2. 75.1 ± 9.0<br />
Total 75.0 ± 9.5<br />
Primary outcomes:<br />
● Entry to institutional care<br />
● Progression of disability<br />
Secondary outcomes:<br />
● BADLS<br />
● MMSE<br />
● NPI<br />
● Carers’ GHQ<br />
● Adverse events<br />
Primary outcomes:<br />
● ADAS-cog<br />
● CIBIC-plus<br />
Secondary outcomes:<br />
● CDR-SB<br />
● IDDD<br />
● Patient-rated QoL<br />
● Adverse events<br />
Primary outcomes:<br />
● CIBIC-plus<br />
Secondary outcomes:<br />
● sMMSE<br />
● Severe Impairment Battery<br />
(SIB)<br />
● DAD<br />
● IADL+<br />
● Physical Self-maintenance<br />
Scale (PSMS+)<br />
● NPI<br />
● Adverse events<br />
Primary outcomes:<br />
● ADAS-cog<br />
Secondary outcomes:<br />
● Explicit verbal recall<br />
(assessed by NYU Stories<br />
Test, delayed recognition<br />
subscale)<br />
● Verbal fluency<br />
● Caregiver-rated global<br />
impression of change<br />
● Compliance<br />
● Adverse events<br />
continued