Donepezil, rivastigmine, galantamine and memantine for ...
Donepezil, rivastigmine, galantamine and memantine for ...
Donepezil, rivastigmine, galantamine and memantine for ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
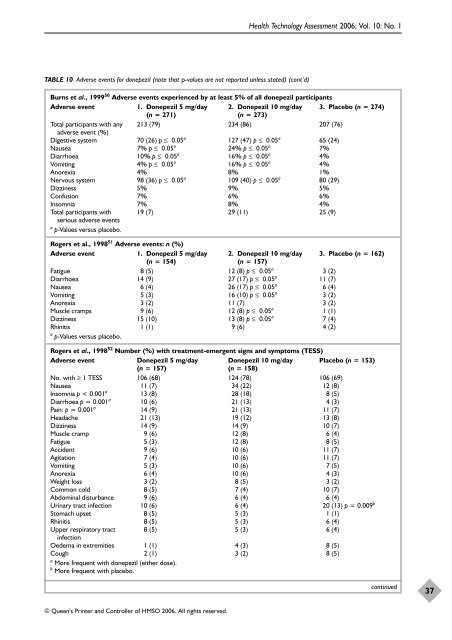
TABLE 10 Adverse events <strong>for</strong> donepezil (note that p-values are not reported unless stated) (cont’d)<br />
© Queen’s Printer <strong>and</strong> Controller of HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 1<br />
Burns et al., 1999 50 Adverse events experienced by at least 5% of all donepezil participants<br />
Adverse event 1. <strong>Donepezil</strong> 5 mg/day 2. <strong>Donepezil</strong> 10 mg/day 3. Placebo (n = 274)<br />
(n = 271) (n = 273)<br />
Total participants with any<br />
adverse event (%)<br />
213 (79) 234 (86) 207 (76)<br />
Digestive system 70 (26) p ≤ 0.05 a<br />
127 (47) p ≤ 0.05 a<br />
65 (24)<br />
Nausea 7% p ≤ 0.05 a<br />
24% p ≤ 0.05 a<br />
7%<br />
Diarrhoea 10% p ≤ 0.05 a<br />
16% p ≤ 0.05 a<br />
4%<br />
Vomiting 4% p ≤ 0.05 a<br />
16% p ≤ 0.05 a<br />
4%<br />
Anorexia 4% 8% 1%<br />
Nervous system 98 (36) p ≤ 0.05 a<br />
109 (40) p ≤ 0.05 a<br />
80 (29)<br />
Dizziness 5% 9% 5%<br />
Confusion 7% 6% 6%<br />
Insomnia 7% 8% 4%<br />
Total participants with 19 (7) 29 (11) 25 (9)<br />
serious adverse events<br />
a p-Values versus placebo.<br />
Rogers et al., 199851 Adverse events: n (%)<br />
Adverse event 1. <strong>Donepezil</strong> 5 mg/day 2. <strong>Donepezil</strong> 10 mg/day 3. Placebo (n = 162)<br />
(n = 154) (n = 157)<br />
Fatigue 8 (5) 12 (8) p ≤ 0.05 a<br />
3 (2)<br />
Diarrhoea 14 (9) 27 (17) p ≤ 0.05 a<br />
11 (7)<br />
Nausea 6 (4) 26 (17) p ≤ 0.05 a<br />
6 (4)<br />
Vomiting 5 (3) 16 (10) p ≤ 0.05 a<br />
3 (2)<br />
Anorexia 3 (2) 11 (7) 3 (2)<br />
Muscle cramps 9 (6) 12 (8) p ≤ 0.05 a<br />
1 (1)<br />
Dizziness 15 (10) 13 (8) p ≤ 0.05 a<br />
Rhinitis 1 (1) 9 (6) 4 (2)<br />
a p-Values versus placebo.<br />
Rogers et al., 199852 Number (%) with treatment-emergent signs <strong>and</strong> symptoms (TESS)<br />
Adverse event <strong>Donepezil</strong> 5 mg/day <strong>Donepezil</strong> 10 mg/day Placebo (n = 153)<br />
(n = 157) (n = 158)<br />
No. with ≥ 1 TESS 106 (68) 124 (78) 106 (69)<br />
Nausea 11 (7) 34 (22) 12 (8)<br />
Insomnia p < 0.001 a<br />
13 (8) 28 (18) 8 (5)<br />
Diarrhoea p = 0.001 a<br />
10 (6) 21 (13) 4 (3)<br />
Pain: p = 0.001 a<br />
7 (4)<br />
14 (9) 21 (13) 11 (7)<br />
Headache 21 (13) 19 (12) 13 (8)<br />
Dizziness 14 (9) 14 (9) 10 (7)<br />
Muscle cramp 9 (6) 12 (8) 6 (4)<br />
Fatigue 5 (3) 12 (8) 8 (5)<br />
Accident 9 (6) 10 (6) 11 (7)<br />
Agitation 7 (4) 10 (6) 11 (7)<br />
Vomiting 5 (3) 10 (6) 7 (5)<br />
Anorexia 6 (4) 10 (6) 4 (3)<br />
Weight loss 3 (2) 8 (5) 3 (2)<br />
Common cold 8 (5) 7 (4) 10 (7)<br />
Abdominal disturbance 9 (6) 6 (4) 6 (4)<br />
Urinary tract infection 10 (6) 6 (4) 20 (13) p = 0.009 b<br />
Stomach upset 8 (5) 5 (3) 1 (1)<br />
Rhinitis 8 (5) 5 (3) 6 (4)<br />
Upper respiratory tract<br />
infection<br />
8 (5) 5 (3) 6 (4)<br />
Oedema in extremities 1 (1) 4 (3) 8 (5)<br />
Cough 2 (1) 3 (2) 8 (5)<br />
a More frequent with donepezil (either dose).<br />
b More frequent with placebo.<br />
continued<br />
37