Donepezil, rivastigmine, galantamine and memantine for ...
Donepezil, rivastigmine, galantamine and memantine for ...
Donepezil, rivastigmine, galantamine and memantine for ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
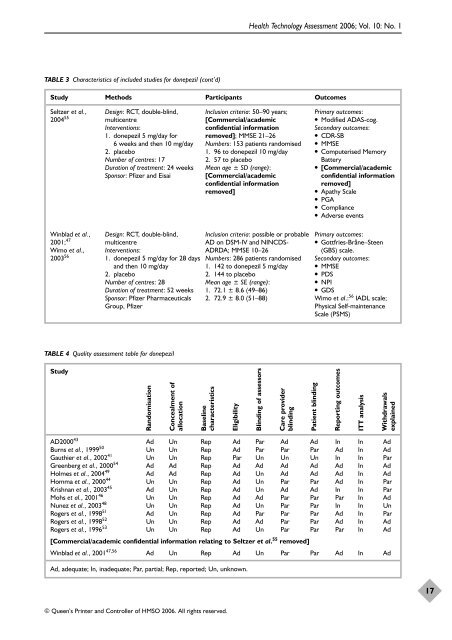
TABLE 3 Characteristics of included studies <strong>for</strong> donepezil (cont’d)<br />
© Queen’s Printer <strong>and</strong> Controller of HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 1<br />
Study Methods Participants Outcomes<br />
Seltzer et al.,<br />
2004 55<br />
Winblad et al.,<br />
2001; 47<br />
Wimo et al.,<br />
2003 56<br />
Design: RCT, double-blind,<br />
multicentre<br />
Interventions:<br />
1. donepezil 5 mg/day <strong>for</strong><br />
6 weeks <strong>and</strong> then 10 mg/day<br />
2. placebo<br />
Number of centres: 17<br />
Duration of treatment: 24 weeks<br />
Sponsor: Pfizer <strong>and</strong> Eisai<br />
Design: RCT, double-blind,<br />
multicentre<br />
Interventions:<br />
1. donepezil 5 mg/day <strong>for</strong> 28 days<br />
<strong>and</strong> then 10 mg/day<br />
2. placebo<br />
Number of centres: 28<br />
Duration of treatment: 52 weeks<br />
Sponsor: Pfizer Pharmaceuticals<br />
Group, Pfizer<br />
TABLE 4 Quality assessment table <strong>for</strong> donepezil<br />
Study<br />
R<strong>and</strong>omisation<br />
Concealment of<br />
allocation<br />
Inclusion criteria: 50–90 years;<br />
[Commercial/academic<br />
confidential in<strong>for</strong>mation<br />
removed]; MMSE 21–26<br />
Numbers: 153 patients r<strong>and</strong>omised<br />
1. 96 to donepezil 10 mg/day<br />
2. 57 to placebo<br />
Mean age ± SD (range):<br />
[Commercial/academic<br />
confidential in<strong>for</strong>mation<br />
removed]<br />
Inclusion criteria: possible or probable<br />
AD on DSM-IV <strong>and</strong> NINCDS-<br />
ADRDA; MMSE 10–26<br />
Numbers: 286 patients r<strong>and</strong>omised<br />
1. 142 to donepezil 5 mg/day<br />
2. 144 to placebo<br />
Mean age ± SE (range):<br />
1. 72.1 ± 8.6 (49–86)<br />
2. 72.9 ± 8.0 (51–88)<br />
Baseline<br />
characteristics<br />
Primary outcomes:<br />
● Modified ADAS-cog.<br />
Secondary outcomes:<br />
● CDR-SB<br />
● MMSE<br />
● Computerised Memory<br />
Battery<br />
● [Commercial/academic<br />
confidential in<strong>for</strong>mation<br />
removed]<br />
● Apathy Scale<br />
● PGA<br />
● Compliance<br />
● Adverse events<br />
Primary outcomes:<br />
● Gottfries-Bråne–Steen<br />
(GBS) scale.<br />
Secondary outcomes:<br />
● MMSE<br />
● PDS<br />
● NPI<br />
● GDS<br />
Wimo et al.: 56 IADL scale;<br />
Physical Self-maintenance<br />
Scale (PSMS)<br />
AD200043 Ad Un Rep Ad Par Ad Ad In In Ad<br />
Burns et al., 1999 50 Un Un Rep Ad Par Par Par Ad In Ad<br />
Gauthier et al., 2002 41 Un Un Rep Par Un Un Un In In Par<br />
Greenberg et al., 2000 54 Ad Ad Rep Ad Ad Ad Ad Ad In Ad<br />
Holmes et al., 2004 49 Ad Ad Rep Ad Un Ad Ad Ad In Ad<br />
Homma et al., 2000 44 Un Un Rep Ad Un Par Par Ad In Par<br />
Krishnan et al., 2003 45 Ad Un Rep Ad Un Ad Ad In In Par<br />
Mohs et al., 2001 46 Un Un Rep Ad Ad Par Par Par In Ad<br />
Nunez et al., 2003 48 Un Un Rep Ad Un Par Par In In Un<br />
Rogers et al., 1998 51 Ad Un Rep Ad Par Par Par Ad In Par<br />
Rogers et al., 1998 52 Un Un Rep Ad Ad Par Par Ad In Ad<br />
Rogers et al., 1996 53 Un Un Rep Ad Un Par Par Par In Ad<br />
[Commercial/academic confidential in<strong>for</strong>mation relating to Seltzer et al. 55 removed]<br />
Winblad et al., 2001 47,56 Ad Un Rep Ad Un Par Par Ad In Ad<br />
Ad, adequate; In, inadequate; Par, partial; Rep, reported; Un, unknown.<br />
Eligibility<br />
Blinding of assessors<br />
Care provider<br />
blinding<br />
Patient blinding<br />
Reporting outcomes<br />
ITT analysis<br />
Withdrawals<br />
explained<br />
17