CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
80<br />
Cl 2<br />
.nH 2<br />
O (n=1-8)<br />
80<br />
Br 2<br />
.nH 2<br />
O (n=1-8)<br />
Energy (kcal/mol)<br />
60<br />
40<br />
20<br />
E solv<br />
E int<br />
Energy (kcal/mol)<br />
60<br />
40<br />
20<br />
E solv<br />
E int<br />
0<br />
0 2 4 6 8<br />
n<br />
0<br />
0 2 4 6 8<br />
n<br />
A<br />
B<br />
Energy (kcal/mol)<br />
80<br />
60<br />
40<br />
20<br />
I 2<br />
.nH 2<br />
O (n=1-8)<br />
E solv<br />
E int<br />
0<br />
0 2 4 6 8<br />
n<br />
C<br />
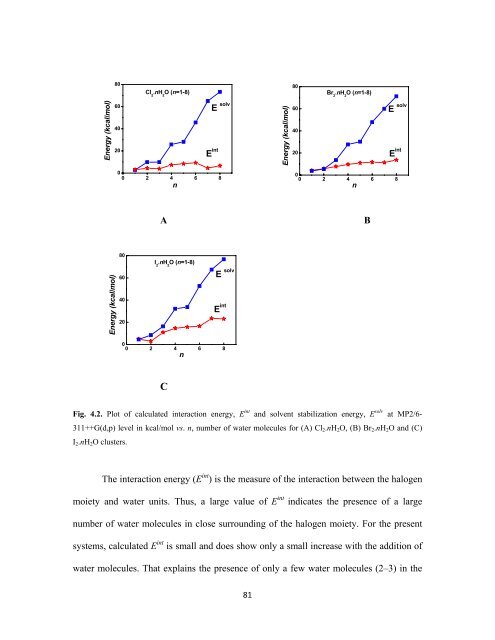
Fig. 4.2. Plot of calculated interaction energy, E int and solvent stabilization energy, E solv at MP2/6-<br />
311++G(d,p) level in kcal/mol vs. n, number of water molecules for (A) Cl 2 .nH 2 O, (B) Br 2 .nH 2 O and (C)<br />
I 2 .nH 2 O clusters.<br />
The interaction energy (E int ) is the measure of the interaction between the halogen<br />
moiety and water units. Thus, a large value of E int indicates the presence of a large<br />
number of water molecules in close surrounding of the halogen moiety. For the present<br />
systems, calculated E int is small and does show only a small increase with the addition of<br />
water molecules. That explains the presence of only a few water molecules (2–3) in the<br />
81