CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
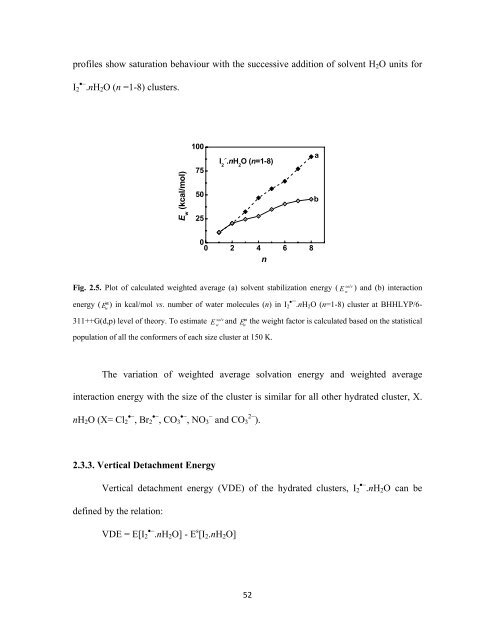
profiles show saturation behaviour with the successive addition of solvent H 2 O units for<br />
I 2 •− .nH 2 O (n =1-8) clusters.<br />
E w<br />
(kcal/mol)<br />
100<br />
75<br />
50<br />
25<br />
I 2 .- .nH 2<br />
O (n=1-8)<br />
a<br />
b<br />
0<br />
0 2 4 6 8<br />
n<br />
solv<br />
Fig. 2.5. Plot of calculated weighted average (a) solvent stabilization energy ( E w<br />
) and (b) interaction<br />
int<br />
energy ( E w<br />
) in kcal/mol vs. number of water molecules (n) in I 2•¯.nH 2 O (n=1-8) cluster at BHHLYP/6-<br />
solv int<br />
311++G(d,p) level of theory. To estimate E w<br />
and E w<br />
the weight factor is calculated based on the statistical<br />
population of all the conformers of each size cluster at 150 K.<br />
The variation of weighted average solvation energy and weighted average<br />
interaction energy with the size of the cluster is similar for all other hydrated cluster, X.<br />
nH 2 O (X= Cl 2 •− , Br 2 •− , CO 3 •− , NO 3 − and CO 3 2− ).<br />
2.3.3. Vertical Detachment Energy<br />
Vertical detachment energy (VDE) of the hydrated clusters, I •− 2 .nH 2 O can be<br />
defined by the relation:<br />
VDE = E[I •− 2 .nH 2 O] - E s [I 2 .nH 2 O]<br />
52