CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
D E F<br />
G<br />
H<br />
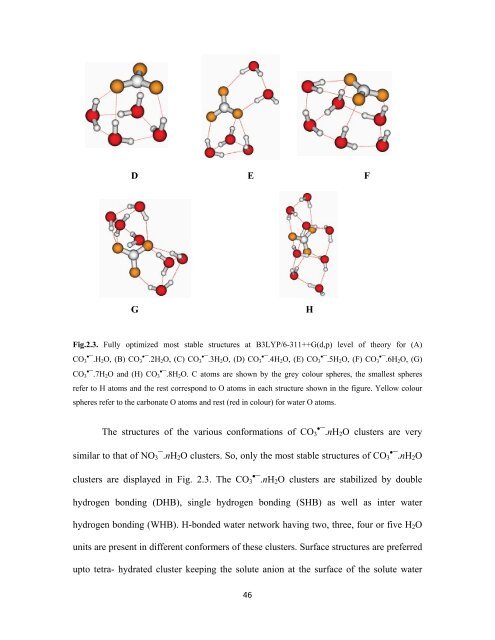
Fig.2.3. Fully optimized most stable structures at B3LYP/6-311++G(d,p) level of theory for (A)<br />
CO 3•¯.H 2 O, (B) CO 3•¯.2H 2 O, (C) CO 3•¯.3H 2 O, (D) CO 3•¯.4H 2 O, (E) CO 3•¯.5H 2 O, (F) CO 3•¯.6H 2 O, (G)<br />
CO 3•¯.7H 2 O and (H) CO 3•¯.8H 2 O. C atoms are shown by the grey colour spheres, the smallest spheres<br />
refer to H atoms and the rest correspond to O atoms in each structure shown in the figure. Yellow colour<br />
spheres refer to the carbonate O atoms and rest (red in colour) for water O atoms.<br />
The structures of the various conformations of CO 3•¯.nH 2 O clusters are very<br />
similar to that of NO 3¯.nH 2 O clusters. So, only the most stable structures of CO 3•¯.nH 2 O<br />
clusters are displayed in Fig. 2.3. The CO 3•¯.nH 2 O clusters are stabilized by double<br />
hydrogen bonding (DHB), single hydrogen bonding (SHB) as well as inter water<br />
hydrogen bonding (WHB). H-bonded water network having two, three, four or five H 2 O<br />
units are present in different conformers of these clusters. Surface structures are preferred<br />
upto tetra- hydrated cluster keeping the solute anion at the surface of the solute water<br />
46