CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
VDE w<br />
(eV)<br />
6.0<br />
5.5<br />
5.0<br />
4.5<br />
I 2 .- .nH 2<br />
O (n=1-8)<br />
E w<br />
(ev)<br />
4<br />
3<br />
2<br />
1<br />
I 2 .- .nH 2<br />
O (n=1-8)<br />
b<br />
a<br />
E w<br />
solv<br />
E w<br />
int<br />
0 2 4 6 8<br />
n<br />
0<br />
4.4 4.8 5.2 5.6 6.0<br />
VDE w<br />
(eV)<br />
I<br />
II<br />
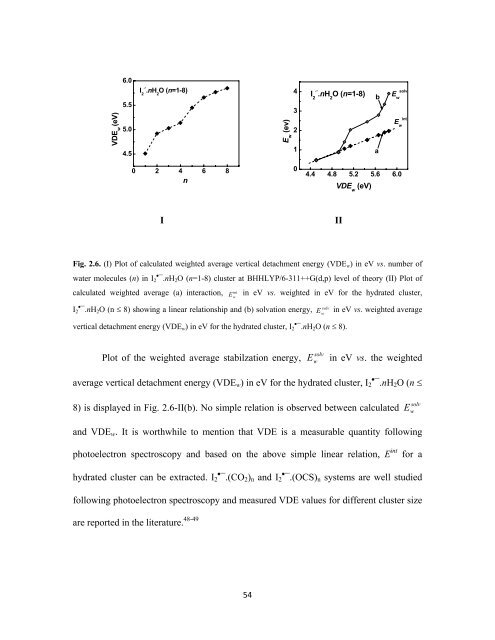
Fig. 2.6. (I) Plot of calculated weighted average vertical detachment energy (VDE w ) in eV vs. number of<br />
water molecules (n) in I 2•¯.nH 2 O (n=1-8) cluster at BHHLYP/6-311++G(d,p) level of theory (II) Plot of<br />
calculated weighted average (a) interaction,<br />
int Ew in eV vs. weighted in eV for the hydrated cluster,<br />
I 2•¯.nH<br />
solv 2 O (n ≤ 8) showing a linear relationship and (b) solvation energy, E w<br />
in eV vs. weighted average<br />
vertical detachment energy (VDE w ) in eV for the hydrated cluster, I 2•¯.nH 2 O (n ≤ 8).<br />
Plot of the weighted average stabilzation energy, E w<br />
solv<br />
in eV vs. the weighted<br />
average vertical detachment energy (VDE w ) in eV for the hydrated cluster, I 2•¯.nH 2 O (n ≤<br />
solv<br />
8) is displayed in Fig. 2.6-II(b). No simple relation is observed between calculated E w<br />
and VDE w . It is worthwhile to mention that VDE is a measurable quantity following<br />
photoelectron spectroscopy and based on the above simple linear relation, E int for a<br />
hydrated cluster can be extracted. I 2•¯.(CO 2 ) n and I 2•¯.(OCS) n systems are well studied<br />
following photoelectron spectroscopy and measured VDE values for different cluster size<br />
are reported in the literature. 48-49<br />
54