CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
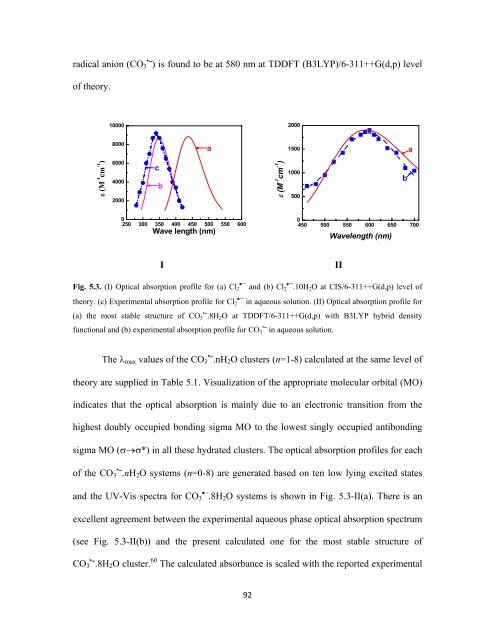
adical anion (CO 3 • ⎯) is found to be at 580 nm at TDDFT (B3LYP)/6-311++G(d,p) level<br />
of theory.<br />
10000<br />
2000<br />
8000<br />
a<br />
1500<br />
a<br />
ε (M -1 cm -1 )<br />
6000<br />
4000<br />
2000<br />
c<br />
b<br />
ε (M -1 cm -1 )<br />
1000<br />
500<br />
b<br />
0<br />
250 300 350 400 450 500 550 600<br />
Wave length (nm)<br />
0<br />
450 500 550 600 650 700<br />
Wavelength (nm)<br />
I<br />
II<br />
Fig. 5.3. (I) Optical absorption profile for (a) Cl 2•¯ and (b) Cl 2•¯.10H 2 O at CIS/6-311++G(d,p) level of<br />
theory. (c) Experimental absorption profile for Cl 2•¯ in aqueous solution. (II) Optical absorption profile for<br />
(a) the most stable structure of CO • 3 ⎯.8H 2 O at TDDFT/6-311++G(d,p) with B3LYP hybrid density<br />
functional and (b) experimental absorption profile for CO • 3 ⎯ in aqueous solution.<br />
The λ max values of the CO • 3 ⎯.nH 2 O clusters (n=1-8) calculated at the same level of<br />
theory are supplied in Table 5.1. Visualization of the appropriate molecular orbital (MO)<br />
indicates that the optical absorption is mainly due to an electronic transition from the<br />
highest doubly occupied bonding sigma MO to the lowest singly occupied antibonding<br />
sigma MO (σ→σ*) in all these hydrated clusters. The optical absorption profiles for each<br />
of the CO • 3 ⎯.nH 2 O systems (n=0-8) are generated based on ten low lying excited states<br />
and the UV-Vis spectra for CO •− 3 .8H 2 O systems is shown in Fig. 5.3-II(a). There is an<br />
excellent agreement between the experimental aqueous phase optical absorption spectrum<br />
(see Fig. 5.3-II(b)) and the present calculated one for the most stable structure of<br />
CO • 3 ⎯.8H 2 O cluster. 60 The calculated absorbance is scaled with the reported experimental<br />
92