CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
CHEM01200604005 A. K. Pathak - Homi Bhabha National Institute
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
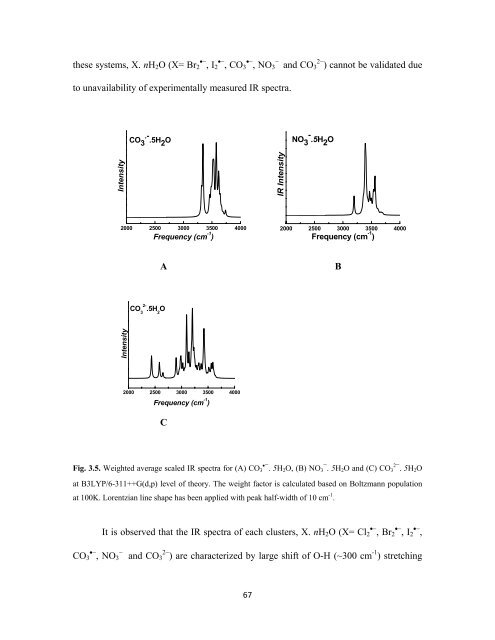
these systems, X. nH 2 O (X= Br 2 •− , I 2 •− , CO 3 •− , NO 3<br />
−<br />
and CO 3 2− ) cannot be validated due<br />
to unavailability of experimentally measured IR spectra.<br />
CO 3<br />
.- .5H2 O<br />
NO 3<br />
- .5H2 O<br />
Intensity<br />
2000 2500 3000 3500 4000<br />
Frequency (cm -1 )<br />
A<br />
B<br />
CO 3 2- .5H 2<br />
O<br />
Intensity<br />
IR Intensity<br />
2000 2500 3000 3500 4000<br />
Frequency (cm -1 )<br />
2000 2500 3000 3500 4000<br />
Frequency (cm -1 )<br />
C<br />
Fig. 3.5. Weighted average scaled IR spectra for (A) CO 3<br />
•− . 5H2 O, (B) NO 3<br />
− . 5H2 O and (C) CO 3<br />
2− . 5H2 O<br />
at B3LYP/6-311++G(d,p) level of theory. The weight factor is calculated based on Boltzmann population<br />
at 100K. Lorentzian line shape has been applied with peak half-width of 10 cm -1 .<br />
It is observed that the IR spectra of each clusters, X. nH 2 O (X= Cl 2 •− , Br 2 •− , I 2 •− ,<br />
CO 3 •− , NO 3<br />
−<br />
and CO 3 2− ) are characterized by large shift of O-H (~300 cm -1 ) stretching<br />
67